An ionophore-based persistent luminescent 'Glow Sensor' for sodium detection
- PMID: 35529711
- PMCID: PMC9073184
- DOI: 10.1039/c9ra05313a
An ionophore-based persistent luminescent 'Glow Sensor' for sodium detection
Abstract
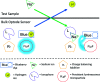
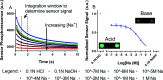
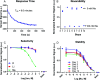
Optical sensors have numerous positive attributes such as low invasiveness, miniaturizability, biocompatibility, and ease of signal transduction. Recently, there has been a strong research focus on using phosphorescent readout mechanisms, specifically from long-lifetime phosphorescent or 'persistent luminescence' particles, for in vitro and in vivo sensors. Persistent luminescence readouts can avoid cellular autofluorescence during biological monitoring, leading to an improved signal-to-noise ratio over a more traditional fluorescence readout. In this study, we show for the first time an ionophore-based optical bulk optode sensor that utilizes persistent luminescence microparticles for ion detection. To achieve this, we combined long-lifetime strontium aluminate-based 'glow-in-the-dark' microparticles with a non-fluorescent pH-responsive dye in a hydrophobic plasticized polymer membrane along with traditional ionophore-based optical sensor components to create a phosphorescent 'Glow Sensor'. The non-fluorescent pH indicator dye gates the strontium aluminate luminescence signal so that it decreases in magnitude with increasing sodium concentration. We characterized the Glow Sensor in terms of emission lifetime, dynamic range, response time, reversibility, selectivity, and stability.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures
Similar articles
-
Persistent Luminescence Nanosensors: A Generalized Optode-Based Platform for Autofluorescence-Free Sensing in Biological Systems.ACS Sens. 2024 Jun 28;9(6):3307-3315. doi: 10.1021/acssensors.4c00653. Epub 2024 Jun 3. ACS Sens. 2024. PMID: 38826054
-
Simple production of photoluminescent polyester coating using lanthanide-doped pigment.Luminescence. 2021 Jun;36(4):1024-1031. doi: 10.1002/bio.4030. Epub 2021 Feb 23. Luminescence. 2021. PMID: 33571395
-
Preparation of flame-retardant, hydrophobic, ultraviolet protective, and luminescent transparent wood.Luminescence. 2021 Dec;36(8):1922-1932. doi: 10.1002/bio.4126. Epub 2021 Aug 9. Luminescence. 2021. PMID: 34323352
-
UV-A,B,C Emitting Persistent Luminescent Materials.Materials (Basel). 2022 Dec 27;16(1):236. doi: 10.3390/ma16010236. Materials (Basel). 2022. PMID: 36614574 Free PMC article. Review.
-
Responsive Metal Complex Probes for Time-Gated Luminescence Biosensing and Imaging.Acc Chem Res. 2020 Jul 21;53(7):1316-1329. doi: 10.1021/acs.accounts.0c00172. Epub 2020 Jun 23. Acc Chem Res. 2020. PMID: 32574043 Review.
Cited by
-
Photoacoustic Chemical Imaging Sodium Nano-Sensor Utilizing a Solvatochromic Dye Transducer for In Vivo Application.Biosensors (Basel). 2023 Oct 11;13(10):923. doi: 10.3390/bios13100923. Biosensors (Basel). 2023. PMID: 37887116 Free PMC article.
-
Nanoparticle-Based Liquid-Liquid Extraction for the Determination of Metal Ions.ACS Sens. 2021 Dec 24;6(12):4408-4416. doi: 10.1021/acssensors.1c01780. Epub 2021 Nov 18. ACS Sens. 2021. PMID: 34793121 Free PMC article.
References
LinkOut - more resources
Full Text Sources

