A rapid on-site analysis method for the simultaneous extraction and determination of Pb2+ and Cd2+ in cereals
- PMID: 35529762
- PMCID: PMC9073185
- DOI: 10.1039/c9ra05587h
A rapid on-site analysis method for the simultaneous extraction and determination of Pb2+ and Cd2+ in cereals
Abstract
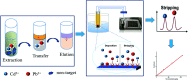
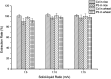
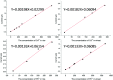
In order to achieve rapid on-site screening and solve the problem of rapid pretreatment for the determination of lead (Pb2+) and cadmium (Cd2+) in cereals by a portable electrochemical analyzer with disposable screen-printed electrodes (SPEs), a new reliable and simple extraction method for Pb2+ and Cd2+ in cereals was developed. The Pb2+ and Cd2+ in cereals were purified by a mixed solution of 1 mol L-1 potassium iodide (KI)/5% vitamin C (VC)/ethyl acetate after being extracted by 10% HNO3, which transfers the Pb2+ and Cd2+ into ethyl acetate after a reaction with KI-VC. Then, the Pb2+ and Cd2+ were eluted from ethyl acetate with 5% HNO3 and were determined by an electrochemical analyzer with screen printed electrodes. Under the optimized conditions, the matrix calibration curves of Pb2+ and Cd2+ in rice and wheat showed good linear relationships with R 2 > 0.996. The method shows a detection limit (LOD) for Cd2+ in rice and wheat of 6.7 μg kg-1 and 11.5 μg kg-1, and the corresponding values for Pb2+ were 34.9 and 31.1 μg kg-1, respectively. The relative standard deviation (RSD) was less than 8.7% for Cd2+ and Pb2+. In addition, the recoveries of the tested reference materials using this method were between 80% and 120%. From sample pretreatment to testing results, the whole process took no more than 25 min, and the operation was simple for operators, green to the environment, cheap in terms of instruments, and above all suitable for on-site detection. The results implied that this portable electrochemical method with new pretreatment may be a good choice for screening Pb2+ and Cd2+ in cereal samples on-site.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





Similar articles
-
Rapid, Simultaneous, and Automatic Determination of Lead and Cadmium in Cereals with a New High Performance Composite Hollow Cathode Lamp Coupled to Graphite Furnace Atomic Absorption Spectrometry.Molecules. 2022 Dec 5;27(23):8571. doi: 10.3390/molecules27238571. Molecules. 2022. PMID: 36500663 Free PMC article.
-
A rapid magnetic-based purification of Cd2+ and Pb2+ prior to portable electrochemical determination for grain.Food Chem X. 2023 Mar 10;18:100636. doi: 10.1016/j.fochx.2023.100636. eCollection 2023 Jun 30. Food Chem X. 2023. PMID: 37008725 Free PMC article.
-
Preparation of electrochemically treated nanoporous pencil-graphite electrodes for the simultaneous determination of Pb and Cd in water samples.Anal Bioanal Chem. 2017 Aug;409(20):4827-4837. doi: 10.1007/s00216-017-0426-3. Epub 2017 Jun 29. Anal Bioanal Chem. 2017. PMID: 28664333
-
Magnetic dispersive solid-phase extraction using ZSM-5 zeolite/Fe2O3 composite coupled with screen-printed electrodes based electrochemical detector for determination of cadmium in urine samples.Talanta. 2020 Dec 1;220:121394. doi: 10.1016/j.talanta.2020.121394. Epub 2020 Jul 15. Talanta. 2020. PMID: 32928414
-
Cadmium and lead in grey wolf liver samples: optimisation of a microwave-assisted digestion method.Arh Hig Rada Toksikol. 2013 Sep;64(3):395-403. doi: 10.2478/10004-1254-64-2013-2323. Arh Hig Rada Toksikol. 2013. PMID: 24084348
Cited by
-
Health Risk Assessment of Exposure to Heavy Metals in Wheat Flour from Iran Markets: Application of Monte Carlo Simulation Approach.Biol Trace Elem Res. 2025 Apr;203(4):2284-2294. doi: 10.1007/s12011-024-04324-z. Epub 2024 Jul 31. Biol Trace Elem Res. 2025. PMID: 39083196
-
Automated and Rapid Easy-to-Use Magnetic Solid-Phase Extraction System for Five Heavy Metals in Cereals and Feeds.Foods. 2022 Dec 7;11(24):3944. doi: 10.3390/foods11243944. Foods. 2022. PMID: 36553685 Free PMC article.
-
Rapid, Simultaneous, and Automatic Determination of Lead and Cadmium in Cereals with a New High Performance Composite Hollow Cathode Lamp Coupled to Graphite Furnace Atomic Absorption Spectrometry.Molecules. 2022 Dec 5;27(23):8571. doi: 10.3390/molecules27238571. Molecules. 2022. PMID: 36500663 Free PMC article.
-
In Situ Deposition of Gold Nanoparticles and L-Cysteine on Screen-Printed Carbon Electrode for Rapid Electrochemical Determination of As(III) in Water and Tea.Biosensors (Basel). 2023 Jan 12;13(1):130. doi: 10.3390/bios13010130. Biosensors (Basel). 2023. PMID: 36671965 Free PMC article.
-
A rapid magnetic-based purification of Cd2+ and Pb2+ prior to portable electrochemical determination for grain.Food Chem X. 2023 Mar 10;18:100636. doi: 10.1016/j.fochx.2023.100636. eCollection 2023 Jun 30. Food Chem X. 2023. PMID: 37008725 Free PMC article.
References
-
- Gumpu M. B. Sethuraman S. Krishnan U. M. Rayappan J. B. B. Sens. Actuators, B. 2015;213:515. doi: 10.1016/j.snb.2015.02.122. - DOI
-
- Chouhan B. Meena P. Poonar N. J. Sci. Eng. Res. 2016;4(4):2015.
LinkOut - more resources
Full Text Sources

