Plasma extracellular vesicle long RNA profiling identifies a diagnostic signature for stage I lung adenocarcinoma
- PMID: 35529788
- PMCID: PMC9073734
- DOI: 10.21037/tlcr-21-729
Plasma extracellular vesicle long RNA profiling identifies a diagnostic signature for stage I lung adenocarcinoma
Abstract
Background: The early diagnosis of lung adenocarcinoma (LUAD) is particularly challenging. Recent studies have reported that extracellular vesicles (EVs) include both small and long RNA. However, the profile and diagnosis-related significance of EV long RNA (exLR) profiles for early LUAD remain unclear.
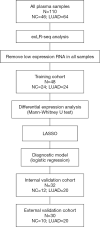
Methods: A case-control analysis was carried out involving 110 participants, including 64 stage I LUAD cases, 24 benign pulmonary nodule (BPN) cases, and 22 healthy controls (HCs). The analysis was performed on the plasma samples' exLR profile based on exLR sequencing. The d-signature was identified using the least absolute shrinkage and selection operator (LASSO) method and a training set (n=48), and validation was completed through use of an internal validation set (n=32) and an external validation set (n=30).
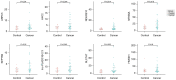
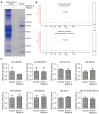
Results: A diagnostic signature (d-signature) encompassing 8 exLR markers (NFKBIA, NDUFB10, SLC7A7, ARPC5, SEPTIN9, HMGN1, H4C2, and lnc-PLA2G1B-2:3) was identified for the detection of LUAD. This d-signature exhibited a high level of accuracy, with an area under the receiver operating characteristic (ROC) curve (AUC) of 0.991 in the training group, 0.921 in the internal validation group, and 0.9 in the external validation group. Moreover, the d-signature could distinguish adenocarcinomas in situ (AIS) and minimally invasive adenocarcinomas (MIA) from the noncancerous controls (NCs), with AUCs of 0.934 and 0.909, respectively, in the combined cohorts.
Conclusions: This study initially characterized the plasma exLR profile of early LUAD and reported on an exLR-based diagnostic signature for the detection of LUAD. This d-signature could be a promising noninvasive biomarker for the early detection and routine screening of LUAD.
Keywords: Extracellular vesicles (EVs); biomarker; diagnostic signature; long RNAs; lung adenocarcinoma (LUAD).
2022 Translational Lung Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-21-729/coif). XL is an employee of Echo Biotech Co., Ltd. The other authors have no conflicts of interest to declare.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous
