Epigenetic Regulation of Host Defense Peptide Synthesis: Synergy Between Histone Deacetylase Inhibitors and DNA/Histone Methyltransferase Inhibitors
- PMID: 35529861
- PMCID: PMC9074817
- DOI: 10.3389/fimmu.2022.874706
Epigenetic Regulation of Host Defense Peptide Synthesis: Synergy Between Histone Deacetylase Inhibitors and DNA/Histone Methyltransferase Inhibitors
Abstract
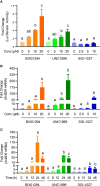
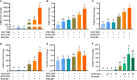
Host defense peptides (HDPs) are an integral part of the innate immune system acting as the first line of defense. Modulation of HDP synthesis has emerged as a promising host-directed approach to fight against infections. Inhibition of histone deacetylation or DNA methylation is known to enhance HDP gene expression. In this study, we explored a possible synergy in HDP gene induction between histone deacetylase inhibitors (HDACi) and DNA/histone methyltransferase inhibitors (DNMTi/HMTi). Two chicken macrophage cell lines were treated with structurally distinct HDACi, HMTi, or DNMTi individually or in combinations, followed by HDP gene expression analysis. Each epigenetic compound was found to be capable of inducing HDP expression. To our surprise, a combination of HDACi and HMTi or HDACi and DNMTi showed a strong synergy to induce the expressions of most HDP genes. The HDP-inducing synergy between butyrate, an HDACi, and BIX01294, an HMTi, were further verified in chicken peripheral blood mononuclear cells. Furthermore, tight junction proteins such as claudin 1 were also synergistically induced by HDACi and HMTi. Overall, we conclude that HDP genes are regulated by epigenetic modifications. Strategies to increase histone acetylation while reducing DNA or histone methylation exert a synergistic effect on HDP induction and, therefore, have potential for the control and prevention of infectious diseases.
Keywords: DNA methyltransferase inhibitors; epigenetic modification; histone deacetylase inhibitors; histone methyltransferase inhibitors; host defense peptides.
Copyright © 2022 Whitmore, Li, Lyu, Khanam and Zhang.
Conflict of interest statement
A provisional patent describing the synergy between histone deacetylase inhibitors and DNA/histone methyltransferase inhibitors was recently filed with the U.S. Patent and Trademark Office with GZ and MW being co-inventors. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

