S100A8 in Serum, Urine, and Saliva as a Potential Biomarker for Systemic Lupus Erythematosus
- PMID: 35529863
- PMCID: PMC9073082
- DOI: 10.3389/fimmu.2022.886209
S100A8 in Serum, Urine, and Saliva as a Potential Biomarker for Systemic Lupus Erythematosus
Abstract
Objectives: This study aimed to elucidate the potential of serum, urine, and saliva S100 calcium-binding protein A8 protein (S100A8) levels as biomarkers for systemic lupus erythematosus (SLE).
Methods: Serum, urine, and saliva samples were obtained from 249 patients with SLE from the Ajou lupus cohort and 52 age- and sex-matched healthy controls (HCs). The concentrations of S100A8 were quantified using an ELISA, and a receiver operating characteristic curve was used to analyze whether they may be used as biomarkers for diagnosing SLE.
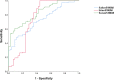
Results: Among 249 SLE patients included in our study, the mean SLE disease activity index (SLEDAI)-2K was 7.16 ± 5.61, and the number of patients with lupus flare was 11. Patients with SLE showed a 2.7-fold increase in serum S100A8 levels compared with that in HCs (1,890.6 vs. 709 pg/ml, p < 0.001). In urine and saliva, the average S100A8 levels were significantly higher in patients with SLE compared with those in HCs (urine, 2,029.4 vs. 1,096.7 pg/ml, p = 0.001; saliva, 290,496.3 vs. 47,742 pg/ml, p < 0.001). For SLE diagnosis, the area under the receiver operating characteristic curve was 0.831 for serum S100A8 (95% CI, 0.765-0.897), 0.751 for urine S100A8 (95% CI, 0.648-0.854), and 0.729 for salivary S100A8 (95% CI, 0.646-0.812). Pearson's correlation analysis showed that S100A8 in serum, urine, and saliva was significantly associated with the SLEDAI (r = 0.267, p < 0.001; r = 0.274, p < 0.001; and r = 0.629, p < 0.001, respectively). Among the clinical manifestations, nephritis was the most influential factor related to SLE in the concentration of S100A8 in serum, urine, and saliva.
Conclusion: This is the first study to show that the expression of S100A8 in serum, urine, and saliva is significantly higher in patients with SLE than in HCs and is associated with disease activity markers. Therefore, we suggest that S100A8 protein could be a potential biomarker for SLE.
Keywords: S100A8; biofluids; biomarkers; disease activity; systemic lupus erythematosus.
Copyright © 2022 Kim, Jung, Lee, Baek, Kim and Suh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
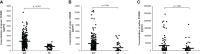

Similar articles
-
Urine and serum S100A8/A9 and S100A12 associate with active lupus nephritis and may predict response to rituximab treatment.RMD Open. 2020 Jul;6(2):e001257. doi: 10.1136/rmdopen-2020-001257. RMD Open. 2020. PMID: 32723832 Free PMC article.
-
Circulating S100 proteins effectively discriminate SLE patients from healthy controls: a cross-sectional study.Rheumatol Int. 2019 Mar;39(3):469-478. doi: 10.1007/s00296-018-4190-2. Epub 2018 Nov 3. Rheumatol Int. 2019. PMID: 30392117
-
Longitudinal assessment of urinary ALCAM, HPX, and PRDX6 in Korean patients with systemic lupus erythematosus: implications for disease activity monitoring and treatment response.Front Immunol. 2024 Jun 10;15:1369385. doi: 10.3389/fimmu.2024.1369385. eCollection 2024. Front Immunol. 2024. PMID: 38915417 Free PMC article.
-
Interferon-Inducible Protein 10 and Disease Activity in Systemic Lupus Erythematosus and Lupus Nephritis: A Systematic Review and Meta-Analysis.Int J Mol Sci. 2019 Oct 8;20(19):4954. doi: 10.3390/ijms20194954. Int J Mol Sci. 2019. PMID: 31597273 Free PMC article.
-
Commentary on the Current Guidelines for the Diagnosis of Lupus Nephritis Flare.Curr Rheumatol Rep. 2019 Feb 27;21(4):12. doi: 10.1007/s11926-019-0809-x. Curr Rheumatol Rep. 2019. PMID: 30810824 Review.
Cited by
-
S-100 Proteins: Basics and Applications as Biomarkers in Animals with Special Focus on Calgranulins (S100A8, A9, and A12).Biology (Basel). 2023 Jun 19;12(6):881. doi: 10.3390/biology12060881. Biology (Basel). 2023. PMID: 37372165 Free PMC article. Review.
-
Proteomic analysis identifies subgroups of patients with active systemic lupus erythematosus.Clin Proteomics. 2023 Jul 29;20(1):29. doi: 10.1186/s12014-023-09420-1. Clin Proteomics. 2023. PMID: 37516862 Free PMC article.
-
Integrative, high-resolution analysis of single cell gene expression across experimental conditions with PARAFAC2-RISE.bioRxiv [Preprint]. 2025 Mar 22:2024.07.29.605698. doi: 10.1101/2024.07.29.605698. bioRxiv. 2025. Update in: Cell Syst. 2025 Jun 18;16(6):101294. doi: 10.1016/j.cels.2025.101294. PMID: 39131377 Free PMC article. Updated. Preprint.
-
Biomarkers for systemic lupus erythematosus: A scoping review.Immun Inflamm Dis. 2024 Oct;12(10):e70022. doi: 10.1002/iid3.70022. Immun Inflamm Dis. 2024. PMID: 39364719 Free PMC article.
-
Changes in S100A8/A9 and S100A12 and Their Comparison with Other Analytes in the Saliva of Pigs with Diarrhea Due to E. coli.Animals (Basel). 2023 Aug 8;13(16):2556. doi: 10.3390/ani13162556. Animals (Basel). 2023. PMID: 37627347 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

