Intranasal Lentiviral Vector-Mediated Antibody Delivery Confers Reduction of SARS-CoV-2 Infection in Elderly and Immunocompromised Mice
- PMID: 35529866
- PMCID: PMC9072863
- DOI: 10.3389/fimmu.2022.819058
Intranasal Lentiviral Vector-Mediated Antibody Delivery Confers Reduction of SARS-CoV-2 Infection in Elderly and Immunocompromised Mice
Abstract
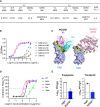
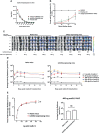
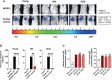
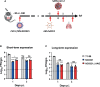
Vaccines for COVID-19 are now a crucial public health need, but the degree of protection provided by conventional vaccinations for individuals with compromised immune systems is unclear. The use of viral vectors to express neutralizing monoclonal antibodies (mAbs) in the lung is an alternative approach that does not wholly depend on individuals having intact immune systems and responses. Here, we identified an anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) monoclonal antibody, NC0321, which can efficiently neutralize a range of SARS-CoV-2 variants, including alpha, beta, delta, and eta. Both prophylactic and therapeutic NC0321 treatments effectively protected mice from SARS-CoV-2 infection. Notably, we adopted viral vector-mediated delivery of NC0321 IgG1 as an attractive approach to prevent SARS-CoV-2 infection. The NC0321 IgG1 expression in the proximal airway, expressed by a single direct in-vivo intranasal (I.N.) administration of a self-inactivating and recombinant lentiviral vector (rSIV.F/HN-NC0321), can protect young, elderly, and immunocompromised mice against mouse-adapted SARS-CoV-2 surrogate challenge. Long-term monitoring indicated that rSIV.F/HN-NC0321 mediated robust IgG expression throughout the airway of young and SCID mice, importantly, no statistical difference in the NC0321 expression between young and SCID mice was observed. A single I.N. dose of rSIV.F/HN-NC0321 30 or 180 days prior to SARS-CoV-2 challenge significantly reduced lung SARS-CoV-2 titers in an Ad5-hACE2-transduced mouse model, reconfirming that this vectored immunoprophylaxis strategy could be useful, especially for those individuals who cannot gain effective immunity from existing vaccines, and could potentially prevent clinical sequelae.
Keywords: COVID-19; SIV.F/HN vectored immunoprophylaxis; monoclonal neutralizing antibody; old and immunodeficient mice; passive immunoprophylaxis.
Copyright © 2022 Du, Zhang, Zhang, Miah, Wei, Zhang, Zhu, Li, Ye, Gill, Hyde, Wang and Zhao.
Conflict of interest statement
DG and SH hold IP in relation to rSIV.F/HN technology. Zhao holds IP in relation to NC0321. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Lung directed antibody gene transfer confers protection against SARS-CoV-2 infection.Thorax. 2022 Dec;77(12):1229-1236. doi: 10.1136/thoraxjnl-2021-217650. Epub 2022 Feb 14. Thorax. 2022. PMID: 35165144 Free PMC article.
-
Vectored immunoprophylaxis and treatment of SARS-CoV-2 infection in a preclinical model.Proc Natl Acad Sci U S A. 2023 Jun 6;120(23):e2303509120. doi: 10.1073/pnas.2303509120. Epub 2023 May 30. Proc Natl Acad Sci U S A. 2023. PMID: 37252952 Free PMC article.
-
SARS-CoV-2 S1 Subunit Booster Vaccination Elicits Robust Humoral Immune Responses in Aged Mice.Microbiol Spectr. 2023 Jun 15;11(3):e0436322. doi: 10.1128/spectrum.04363-22. Epub 2023 May 10. Microbiol Spectr. 2023. PMID: 37162333 Free PMC article.
-
Intranasal administration of adenoviral vaccines expressing SARS-CoV-2 spike protein improves vaccine immunity in mouse models.Vaccine. 2023 May 11;41(20):3233-3246. doi: 10.1016/j.vaccine.2023.04.020. Epub 2023 Apr 14. Vaccine. 2023. PMID: 37085458 Free PMC article.
-
Intranasal Delivery of MVA Vector Vaccine Induces Effective Pulmonary Immunity Against SARS-CoV-2 in Rodents.Front Immunol. 2021 Nov 11;12:772240. doi: 10.3389/fimmu.2021.772240. eCollection 2021. Front Immunol. 2021. PMID: 34858430 Free PMC article.
Cited by
-
Rapid Development of Small Rodent Animal Models for Infectious Disease Research Through Vectorized Receptor Molecule Expression.Viruses. 2024 Nov 19;16(11):1794. doi: 10.3390/v16111794. Viruses. 2024. PMID: 39599908 Free PMC article. Review.
-
Prospects of animal models and their application in studies on adaptive immunity to SARS-CoV-2.Front Immunol. 2022 Sep 16;13:993754. doi: 10.3389/fimmu.2022.993754. eCollection 2022. Front Immunol. 2022. PMID: 36189203 Free PMC article. Review.
References
-
- Kennedy NA, Lin S, Goodhand JR, Chanchlani N, Hamilton B, Bewshea C, et al. . Contributors to the, Infliximab Is Associated With Attenuated Immunogenicity to BNT162b2 and ChAdOx1 Ncov–19 SARS–CoV–2 Vaccines in Patients With IBD. Gut (2021) 70(10):1884–93. doi: 10.1101/2021.03.25.21254335 - DOI - PubMed
-
- Hughes K, Middleton DB, Nowalk MP, Balasubramani GK, Martin ETM, Gaglani HK, et al. . Effectiveness of Influenza Vaccine for Preventing Laboratory–Confirmed Influenza Hospitalizations in Immunocompromised Adults. Clin Infect Dis (2021) 73(11):e4353–60. doi: 10.1101/2020.10.08.20208579 - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

