Cochlear Marginal Cell Pyroptosis Is Induced by Cisplatin via NLRP3 Inflammasome Activation
- PMID: 35529876
- PMCID: PMC9067579
- DOI: 10.3389/fimmu.2022.823439
Cochlear Marginal Cell Pyroptosis Is Induced by Cisplatin via NLRP3 Inflammasome Activation
Abstract
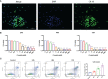
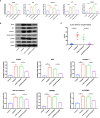
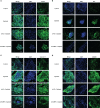
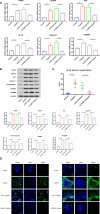
Better understanding the mechanism of cisplatin-induced ototoxicity is of great significance for clinical prevention and treatment of cisplatin-related hearing loss. However, the mechanism of cisplatin-induced inflammatory response in cochlear stria vascularis and the mechanism of marginal cell (MC) damage have not been fully clarified. In this study, a stable model of cisplatin-induced MC damage was established in vitro, and the results of PCR and Western blotting showed increased expressions of NLRP3, Caspase-1, IL-1β, and GSDMD in MCs. Incomplete cell membranes including many small pores appearing on the membrane were also observed under transmission electron microscopy and scanning electron microscopy. In addition, downregulation of NLRP3 by small interfering RNA can alleviate cisplatin-induced MC pyroptosis, and reducing the expression level of TXNIP possesses the inhibition effect on NLRP3 inflammasome activation and its mediated pyroptosis. Taken together, our results suggest that NLRP3 inflammasome activation may mediate cisplatin-induced MC pyroptosis in cochlear stria vascularis, and TXNIP is a possible upstream regulator, which may be a promising therapeutic target for alleviating cisplatin-induced hearing loss.
Keywords: NLRP3; TXNIP; cisplatin; marginal cells; pyroptosis.
Copyright © 2022 Yu, Zong, Zhou, Wei, Wang, Ming and Xiao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Oridonin alleviates kanamycin-related hearing loss by inhibiting NLRP3/caspase-1/gasdermin D-induced inflammasome activation and hair cell pyroptosis.Mol Immunol. 2022 Sep;149:66-76. doi: 10.1016/j.molimm.2022.06.006. Epub 2022 Jun 21. Mol Immunol. 2022. PMID: 35749835
-
Antcin A alleviates pyroptosis and inflammatory response in Kupffercells of non-alcoholic fatty liver disease by targeting NLRP3.Int Immunopharmacol. 2021 Nov;100:108126. doi: 10.1016/j.intimp.2021.108126. Epub 2021 Sep 4. Int Immunopharmacol. 2021. PMID: 34492534
-
Suppression of NLRP3 Inflammasome, Pyroptosis, and Cell Death by NIM811 in Rotenone-Exposed Cells as an in vitro Model of Parkinson's Disease.Neurodegener Dis. 2020;20(2-3):73-83. doi: 10.1159/000511207. Epub 2020 Nov 11. Neurodegener Dis. 2020. PMID: 33176317
-
NLRP3 Inflammasome/Pyroptosis: A Key Driving Force in Diabetic Cardiomyopathy.Int J Mol Sci. 2022 Sep 13;23(18):10632. doi: 10.3390/ijms231810632. Int J Mol Sci. 2022. PMID: 36142531 Free PMC article. Review.
-
The role of mitochondria in NLRP3 inflammasome activation.Mol Immunol. 2018 Nov;103:115-124. doi: 10.1016/j.molimm.2018.09.010. Epub 2018 Sep 21. Mol Immunol. 2018. PMID: 30248487 Review.
Cited by
-
Mitochondrial dysfunction in hearing loss: Oxidative stress, autophagy and NLRP3 inflammasome.Front Cell Dev Biol. 2023 Feb 20;11:1119773. doi: 10.3389/fcell.2023.1119773. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36891515 Free PMC article. Review.
-
Single-Cell Sequencing Reveals Circadian Sensitivity of Noise-Induced Hearing Loss Mediated by Macrophage-Driven NLRP3 Inflammasome Activation.Neurosci Bull. 2025 Jul 20. doi: 10.1007/s12264-025-01440-1. Online ahead of print. Neurosci Bull. 2025. PMID: 40684422
-
Molecular Characteristics of Cisplatin-Induced Ototoxicity and Therapeutic Interventions.Int J Mol Sci. 2023 Nov 20;24(22):16545. doi: 10.3390/ijms242216545. Int J Mol Sci. 2023. PMID: 38003734 Free PMC article. Review.
-
Silencing of TXNIP attenuates oxidative stress injury in HEI-OC1 by inhibiting the activation of NLRP3 and NF-κB.Heliyon. 2024 Sep 10;10(18):e37753. doi: 10.1016/j.heliyon.2024.e37753. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39381226 Free PMC article.
-
Melatonin Ameliorates Senescence of Mouse Auditory Cell Line HEI-OC1 Cells by Suppressing NLRP3 Inflammasome-Mediated Pyroptosis.Mol Neurobiol. 2025 Aug;62(8):9902-9915. doi: 10.1007/s12035-025-04880-y. Epub 2025 Apr 2. Mol Neurobiol. 2025. PMID: 40169516 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

