β2-Integrins - Regulatory and Executive Bridges in the Signaling Network Controlling Leukocyte Trafficking and Migration
- PMID: 35529883
- PMCID: PMC9072638
- DOI: 10.3389/fimmu.2022.809590
β2-Integrins - Regulatory and Executive Bridges in the Signaling Network Controlling Leukocyte Trafficking and Migration
Abstract
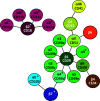
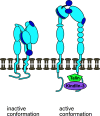
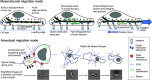
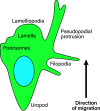
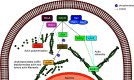
Leukocyte trafficking is an essential process of immunity, occurring as leukocytes travel within the bloodstream and as leukocyte migration within tissues. While it is now established that leukocytes can utilize the mesenchymal migration mode or amoeboid migration mode, differences in the migratory behavior of leukocyte subclasses and how these are realized on a molecular level in each subclass is not fully understood. To outline these differences, first migration modes and their dependence on parameters of the extracellular environments will be explained, as well as the intracellular molecular machinery that powers migration in general. Extracellular parameters are detected by adhesion receptors such as integrins. β2-integrins are surface receptors exclusively expressed on leukocytes and are essential for leukocytes exiting the bloodstream, as well as in mesenchymal migration modes, however, integrins are dispensable for the amoeboid migration mode. Additionally, the balance of different RhoGTPases - which are downstream of surface receptor signaling, including integrins - mediate formation of membrane structures as well as actin dynamics. Individual leukocyte subpopulations have been shown to express distinct RhoGTPase profiles along with their differences in migration behavior, which will be outlined. Emerging aspects of leukocyte migration include signal transduction from integrins via actin to the nucleus that regulates DNA status, gene expression profiles and ultimately leukocyte migratory phenotypes, as well as altered leukocyte migration in tumors, which will be touched upon.
Keywords: LFA-1; RhoGTPases; actin tread milling; amoeboid migration; epigenetics; leukocyte migration; β2-integrins.
Copyright © 2022 Guenther.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

