Cs2CO3-Promoted reaction of tertiary bromopropargylic alcohols and phenols in DMF: a novel approach to α-phenoxyketones
- PMID: 35529892
- PMCID: PMC9039521
- DOI: 10.3762/bjoc.18.44
Cs2CO3-Promoted reaction of tertiary bromopropargylic alcohols and phenols in DMF: a novel approach to α-phenoxyketones
Abstract
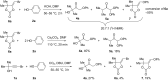
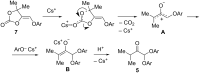
The reaction of bromopropargylic alcohols with phenols in the presence of Cs2CO3/DMF affords α-phenoxy-α'-hydroxyketones (1:1 adducts) and α,α-diphenoxyketones (1:2 adducts) in up to 92% and 24% yields, respectively. Both products are formed via ring opening of the same intermediates, 1,3-dioxolan-2-ones, generated in situ from bromopropargylic alcohols and Cs2CO3.
Keywords: 1,3-dioxolan-2-one; acetylenic alcohol; bromoacetylene; phenols; phenoxyketone.
Copyright © 2022, Volostnykh et al.
Figures


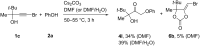
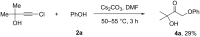




Similar articles
-
Manganese(I)-Catalyzed Cross-Coupling of Ketones and Secondary Alcohols with Primary Alcohols.ACS Omega. 2019 Jun 20;4(6):10741-10754. doi: 10.1021/acsomega.9b01246. eCollection 2019 Jun 30. ACS Omega. 2019. PMID: 31460172 Free PMC article.
-
Nucleophilic addition of phenol derivatives to methyl 1-nitrocyclopropanecarboxylates.J Org Chem. 2008 Sep 5;73(17):6838-40. doi: 10.1021/jo8010705. Epub 2008 Aug 1. J Org Chem. 2008. PMID: 18671432
-
A convenient approach to β-heteroarylated (C-N bond) ketones from Cs2CO3 promoted reaction between propargyl alcohols and nitrogen-heterocycles.Org Biomol Chem. 2012 May 7;10(17):3538-55. doi: 10.1039/c2ob25165e. Epub 2012 Mar 23. Org Biomol Chem. 2012. PMID: 22441201
-
Synthesis of Quinolines via a Metal-Catalyzed Dehydrogenative N-Heterocyclization.Chem Rec. 2017 Feb;17(2):200-216. doi: 10.1002/tcr.201600083. Epub 2016 Aug 15. Chem Rec. 2017. PMID: 27524555 Review.
-
Synthesis and Application of the Transition Metal Complexes of α-Pyridinyl Alcohols, α-Bipyridinyl Alcohols, α,α'-Pyridinyl Diols and α,α'-Bipyridinyl Diols in Homogeneous Catalysis.Molecules. 2018 Apr 12;23(4):896. doi: 10.3390/molecules23040896. Molecules. 2018. PMID: 29649178 Free PMC article. Review.
References
-
- Wang S, Yu L, Li P, Meng L, Wang L. Synthesis. 2011:1541–1546. doi: 10.1055/s-0030-1259998. - DOI
-
- de Orbe M E, Zanini M, Quinonero O, Echavarren A M. ACS Catal. 2019;9:7817–7822. doi: 10.1021/acscatal.9b02314. - DOI
-
- Krishnan K K, Ujwaldev S M, Thankachan A P, Harry N A, Gopinathan A. Mol Catal. 2017;440:140–147. doi: 10.1016/j.mcat.2017.07.021. - DOI
LinkOut - more resources
Full Text Sources
