Menadione: a platform and a target to valuable compounds synthesis
- PMID: 35529893
- PMCID: PMC9039524
- DOI: 10.3762/bjoc.18.43
Menadione: a platform and a target to valuable compounds synthesis
Abstract
Naphthoquinones are important natural or synthetic compounds belonging to the general class of quinones. Many compounds in this class have become drugs that are on the pharmaceutical market for the treatment of various diseases. A special naphthoquinone derivative is menadione, a synthetic naphthoquinone belonging to the vitamin K group. This compound can be synthesized by different methods and it has a broad range of biological and synthetic applications, which will be highlighted in this review.
Keywords: 2-methyl-1,4-naphthoquinone; cancer; chemical reactions; quinone; synthetic platform; vitamin K.
Copyright © 2022, de Souza et al.
Figures



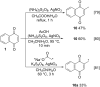

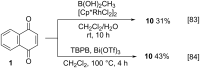
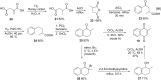
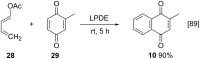
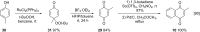
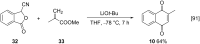








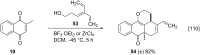


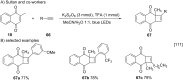


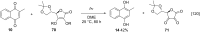

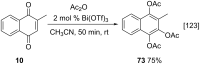
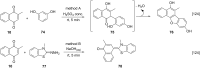

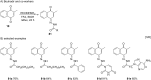



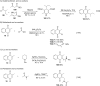
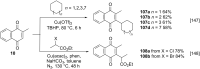
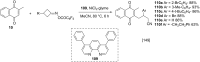

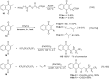

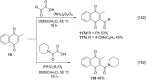
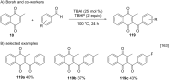
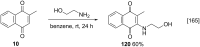
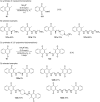
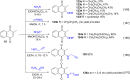


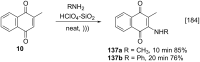
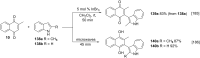
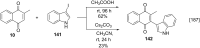





References
-
- de Carvalho da Silva F, Francisco Ferreira V. Curr Org Synth. 2016;13(3):334–371. doi: 10.2174/1570179412666150817220343. - DOI
-
- Goodwin T W, Mercer E I. Introduction to plant Biochemistry. Pergamon Press; 1972.
Publication types
LinkOut - more resources
Full Text Sources
