An ultra-sensitive label-free electrochemiluminescence CKMB immunosensor using a novel nanocomposite-modified printed electrode
- PMID: 35529968
- PMCID: PMC9074035
- DOI: 10.1039/c9ra05016g
An ultra-sensitive label-free electrochemiluminescence CKMB immunosensor using a novel nanocomposite-modified printed electrode
Abstract
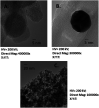
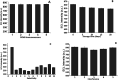
This study presents a novel and ultrasensitive electrochemiluminescence approach for the quantitative assessment of creatine kinase MB (CK-MB). Both carbon, carbon nano-onions (CNOs) and metal-based nanoparticles, such as gold nanoparticles (AuNPs) and iron oxide (Fe3O4), were combined to generate a unique nanocomposite for the detection of CKMB. The immunosensor construction involved the deposition of the nanocomposite on the working electrode, followed by the incubation of an antibody and a blocking agent. Tris(2,2'-bipyridyl)-ruthenium(ii) chloride ([Ru(bpy)3]2+Cl) was used as a luminophore, where tri-n-propylamine (TPrA) was selected as the co-reactant due to its aqueous immobility and luminescence properties. The analytical performance was demonstrated by cyclic voltammetry on ECL. The characterization of each absorbed layer was performed by cyclic voltammetry (CV) and chronocoulometry (CC) techniques in both EC and ECL. For further characterization of iron oxide, gold nanoparticles and carbon nano-onions, scanning electron microscopy (SEM), transmission electron microscopy (TEM) and X-ray diffraction (XRD) were performed. The proposed immunosensor showcases a wide linear range (10 ng mL-1 to 50 fg mL-1), with an extremely low limit of detection (5 fg mL-1). This CKMB immunosensor also exhibits remarkable selectivity, reproducibility, stability and resistance capability towards common interferences available in human serum. In addition, the immunosensor holds great potential to work with real serum samples for clinical diagnosis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no financial or commercial conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

