Ru(ii)-N-heterocyclic carbene complexes: synthesis, characterization, transfer hydrogenation reactions and biological determination
- PMID: 35529977
- PMCID: PMC9074002
- DOI: 10.1039/c9ra05605j
Ru(ii)-N-heterocyclic carbene complexes: synthesis, characterization, transfer hydrogenation reactions and biological determination
Abstract
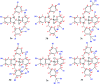
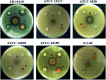
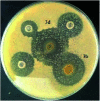
A series of ruthenium(ii) complexes with N-heterocyclic carbene ligands were successfully synthesized by transmetalation reactions between silver(i) N-heterocyclic carbene complexes and [RuCl2(p-cymene)]2 in dichloromethane under Ar conditions. All new compounds were characterized by spectroscopic and analytical methods. These ruthenium(ii)-NHC complexes were found to be efficient precatalysts for the transfer hydrogenation of ketones by using 2-propanol as the hydrogen source in the presence of KOH as a co-catalyst. The antibacterial activity of ruthenium N-heterocyclic carbene complexes 3a-f was measured by disc diffusion method against Gram positive and Gram-negative bacteria. Compounds 3d exhibited potential antibacterial activity against five bacterial species among the six used as indicator cells. The product 3e inhibits the growth of all the six tested microorganisms. Moreover, the antioxidant activity determination of these complexes 3a-f, using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2'-azinobis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS) as reagent, showed that compounds 3b and 3d possess DPPH and ABTS antiradical activities. From a concentration of 1 mg ml-1, these two complexes presented a similar scavenging activity to that of the two used controls gallic acid (GA) and butylated hydroxytoluene (BHT). From a concentration of 10 mg ml-1, the percentage inhibition of complexes 3b and 3d was respectively 70% and 90%. In addition, these two Ru-NHC complexes exhibited antifungal activity against Candida albicans. Investigation of the anti-acetylcholinesterase activity of the studied complexes showed that compounds 3a, 3b, 3d and 3e exhibited good activity at 100 μg ml-1 and product 3d is the most active. In a cytotoxicity study the complexes 3 were evaluated against two human cancer cell lines MDA-MB-231 and MCF-7. Both 3d and 3e complexes were found to be active against the tested cell lines showing comparable activity with examples in the literature.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







References
-
- Zassinovich G. Mestroni G. Gladiali S. Chem. Rev. 1992;92:1051. doi: 10.1021/cr00013a015. - DOI
-
- de Graauw C. F. Peters J. A. van Bekkum H. Husken J. Synthesis. 1994;10:1007. doi: 10.1055/s-1994-25625. - DOI
-
- Noyori R. Hashigushi S. Acc. Chem. Res. 1997;30:97. doi: 10.1021/ar9502341. - DOI
-
- Yamakawa M. Ito H. Noyori R. J. Am. Chem. Soc. 2000;122:1466. doi: 10.1021/ja991638h. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

