Normal vibrations of ternary DOPC/DPPC/cholesterol lipid bilayers by low-frequency Raman spectroscopy
- PMID: 35530012
- PMCID: PMC9073921
- DOI: 10.1039/c9ra06114b
Normal vibrations of ternary DOPC/DPPC/cholesterol lipid bilayers by low-frequency Raman spectroscopy
Abstract
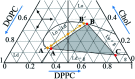
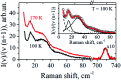
A lipid bilayer containing a ternary mixture of low- and high-melting lipids and cholesterol (Chol) can give rise to domain formation, referred to as lipid rafts. Low-frequency Raman spectroscopy at reduced temperatures allows detection of normal membrane mechanical vibrations. In this work, Raman spectra were obtained in the spectral range between 5 and 90 cm-1 for bilayers prepared from dioleoyl-glycero-phosphocholine (DOPC), dipalmitoyl-glycero-phosphocholine (DPPC) and Chol. A narrow peak detected between 13 and 16 cm-1 was attributed to the vibrational eigenmode of a lipid monolayer (a leaflet). For the equimolar DOPC/DPPC ratio, the Chol concentration dependence for the peak position, width and amplitude may be divided into three distinct ranges: below 9 mol%, the intermediate range between 9 mol% and 38 mol%, and above 38 mol%. In the intermediate range the peak position attains its minimum, and the peak width drops approximately by a factor of two as compared with the Chol-free bilayers. Meanwhile, this range is known for raft formation in a fluid state. The obtained results may be interpreted as evidence that bilayer structures in the raft-containing fluid state may be frozen at low temperatures. The drop of peak width indicates that at the spatial scale of the experiment (∼2.5 nm) the intermolecular bilayer structure with raft formation becomes more homogeneous and more cohesive.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Juhasz J. Sharom F. J. Davis J. H. Quantitative characterization of coexisting phases in DOPC/DPPC/cholesterol mixtures: comparing confocal fluorescence microscopy and deuterium nuclear magnetic resonance. Biochim. Biophys. Acta. 2009;1788:2541–2552. doi: 10.1016/j.bbamem.2009.10.006. - DOI - PubMed
LinkOut - more resources
Full Text Sources

