A Home-Treatment Algorithm Based on Anti-inflammatory Drugs to Prevent Hospitalization of Patients With Early COVID-19: A Matched-Cohort Study (COVER 2)
- PMID: 35530041
- PMCID: PMC9073076
- DOI: 10.3389/fmed.2022.785785
A Home-Treatment Algorithm Based on Anti-inflammatory Drugs to Prevent Hospitalization of Patients With Early COVID-19: A Matched-Cohort Study (COVER 2)
Abstract
Background and aim: While considerable success has been achieved in the management of patients hospitalized with severe coronavirus disease 2019 (COVID-19), far less progress has been made with early outpatient treatment. We assessed whether the implementation of a home treatment algorithm-designed based on a pathophysiologic and pharmacologic rationale-and including non-steroidal anti-inflammatory drugs, especially relatively selective cyclooxygenase-2 inhibitors and, when needed, corticosteroids, anticoagulants, oxygen therapy and antibiotics-at the very onset of mild COVID-19 symptoms could effectively reduce hospital admissions.
Methods: This fully academic, matched-cohort study evaluated outcomes in 108 consecutive consenting patients with mild COVID-19, managed at home by their family doctors between January 2021 and May 2021, according to the proposed treatment algorithm and in 108 age-, sex-, and comorbidities-matched patients on other therapeutic schedules (ClinicalTrials.gov: NCT04854824). The primary outcome was COVID-19-related hospitalization. Analyses were by intention-to-treat.
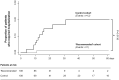
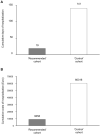
Results: One (0.9%) patient in the "recommended" cohort and 12 (11.1%) in the "control" cohort were admitted to hospital (P = 0.0136). The proposed algorithm reduced the cumulative length of hospital stays by 85% (from 141 to 19 days) as well as related costs (from €60.316 to €9.058). Only 9.8 patients needed to be treated with the recommended algorithm to prevent one hospitalization event. The rate of resolution of major symptoms was numerically-but not significantly-higher in the "recommended" than in the "control" cohort (97.2 vs. 93.5%, respectively; P = 0.322). Other symptoms lingered in a smaller proportion of patients in the "recommended" than in the "control" cohort (20.4 vs. 63.9%, respectively; P < 0.001), and for a shorter period.
Conclusion: The adoption of the proposed outpatient treatment algorithm during the early, mild phase of COVID-19 reduced the incidence of subsequent hospitalization and related costs.
Keywords: COVID-19; SARS-CoV-2; at home management; hospitalization; outpatients.
Copyright © 2022 Consolaro, Suter, Rubis, Pedroni, Moroni, Pastò, Paganini, Pravettoni, Cantarelli, Perico, Perna, Peracchi, Ruggenenti and Remuzzi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Johns, Hopkins CSSE . COVID-19 Map - Johns Hopkins Coronavirus Resource Center. Available online at: https://www.coronavirus.jhu.edu (accessed September 1, 2021).
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

