Transition metal catalyzed [6 + 2] cycloadditions
- PMID: 35530050
- PMCID: PMC9070306
- DOI: 10.1039/c9ra02839k
Transition metal catalyzed [6 + 2] cycloadditions
Abstract
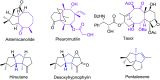
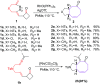
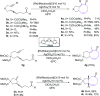
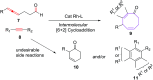
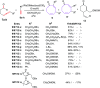
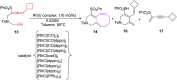
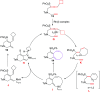
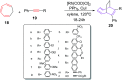
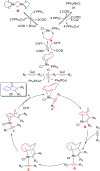
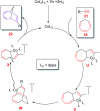
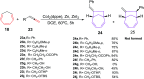
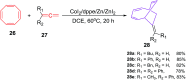
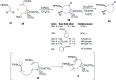
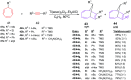
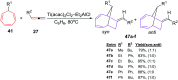
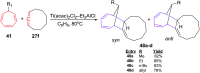
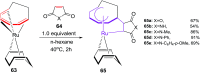
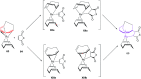
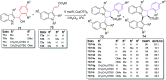
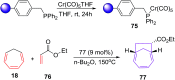
The [6 + 2] cycloaddition reactions are one of the important synthetic transformations to construct eight membered carbo-/heterocyclic systems. The present review is an attempt to update readers on transition metal catalyzed [6 + 2] cycloaddition reactions of various 6π-contributing substrates such as cycloheptatrienes (CHT), cyclooctatetrenes (COTT), allenals, vinylcyclobutanones, fulvene etc. employing rhodium, cobalt, titanium, copper, platinum, ruthenium, rhenium and diphenylprolinolsilyl ethers etc. as catalysts. The transition metal catalyzed [6 + 2] cycloaddition reactions with a variety of functionalized substrates provide straightforward access to eight membered cyclic and/or 5/8, 6/8 etc. condensed carbo-/heterocyclic molecules in moderate to good yields.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
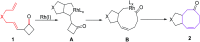

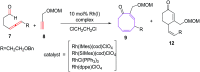
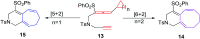

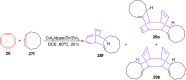

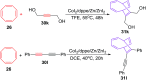

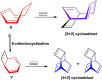
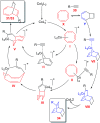

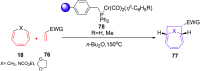
References
-
- Advances in Cycloaddition, ed. D. P. Curran, JAI Press, Greenwich, 1994, vol. 1–3
- Trost B. M. Angew. Chem., Int. Ed. Engl. 1995;34:259. doi: 10.1002/anie.199502591. - DOI
- Lautens M. Klute W. Tam W. Chem. Rev. 1996;96:49. doi: 10.1021/cr950016l. - DOI - PubMed
- Schore N. E. Chem. Rev. 1988;88:1081. doi: 10.1021/cr00089a006. - DOI
-
-
[5+2] cycloaddition references see:
- Pellissiera H. Adv. Synth. Catal. 2011;353:189. doi: 10.1002/adsc.201000695. - DOI
-
and reference cited therein
-
Publication types
LinkOut - more resources
Full Text Sources

