Bioceramic akermanite enhanced vascularization and osteogenic differentiation of human induced pluripotent stem cells in 3D scaffolds in vitro and vivo
- PMID: 35530104
- PMCID: PMC9070079
- DOI: 10.1039/c9ra02026h
Bioceramic akermanite enhanced vascularization and osteogenic differentiation of human induced pluripotent stem cells in 3D scaffolds in vitro and vivo
Abstract
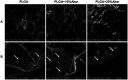
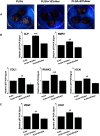
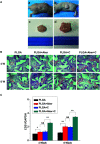
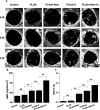
A growing number of studies suggest that the modulation of cell differentiation by biomaterials is critical for tissue engineering. In previous work, we demonstrated that human induced pluripotent stem cells (iPSCs) are remarkably promising seed cells for bone tissue engineering. In addition, we found that the ionic products of akermanite (Aker) are potential inducers of osteogenic differentiation of iPSCs. Furthermore, composite scaffolds containing polymer and bioceramics have more interesting properties compared to pure bioceramic scaffolds for bone tissue engineering. The characteristic of model biomaterials in bone tissue engineering is their ability to control the osteogenic differentiation of stem cells and simultaneously induce the angiogenesis of endothelia cells. Thus, this study aimed at investigating the effects of poly(lactic-co-glycolic acid)/Aker (PLGA-Aker) composite scaffolds on angiogenic and osteogenic differentiation of human iPSCs in order to optimize the scaffold compositions. The results from Alizarin Red S staining, qRT-PCR analysis of osteogenic genes (BMP2, RUNX2, ALP, COL1 and OCN) and angiogenic genes (VEGF and CD31) demonstrated that PLGA/Aker composite scaffolds containing 10% Aker exhibited the highest stimulatory effects on the osteogenic and angiogenic differentiation of human iPSCs among all scaffolds. After the scaffolds were implanted in nu/nu mice subcutaneous pockets and calvarial defects, H&E staining, BSP immunostaining, qRT-PCR analysis and micro-CT analysis (BMD, BV/TV) indicated that PLGA + 10% Aker scaffolds enhanced the vascularization and osteogenic differentiation of human iPSCs and stimulated the repair of bone defects. Taken together, our work indicated that combining scaffolds containing silicate bioceramic Aker and human iPSCs is a promising approach for the enhancement of bone regeneration.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
The stimulation of osteogenic differentiation of embryoid bodies from human induced pluripotent stem cells by akermanite bioceramics.J Mater Chem B. 2016 Apr 7;4(13):2369-2376. doi: 10.1039/c6tb00398b. Epub 2016 Mar 16. J Mater Chem B. 2016. PMID: 32263232
-
Supercritical CO2 foamed composite scaffolds incorporating bioactive lipids promote vascularized bone regeneration via Hif-1α upregulation and enhanced type H vessel formation.Acta Biomater. 2019 Aug;94:253-267. doi: 10.1016/j.actbio.2019.05.066. Epub 2019 May 31. Acta Biomater. 2019. PMID: 31154054
-
Silicate bioceramics enhanced vascularization and osteogenesis through stimulating interactions between endothelia cells and bone marrow stromal cells.Biomaterials. 2014 Apr;35(12):3803-18. doi: 10.1016/j.biomaterials.2014.01.039. Epub 2014 Jan 31. Biomaterials. 2014. PMID: 24486216
-
Human urine-derived stem cells can be induced into osteogenic lineage by silicate bioceramics via activation of the Wnt/β-catenin signaling pathway.Biomaterials. 2015 Jul;55:1-11. doi: 10.1016/j.biomaterials.2015.03.029. Epub 2015 Apr 5. Biomaterials. 2015. PMID: 25934447
-
Angiogenic and osteogenic potentials of dental stem cells in bone tissue engineering.J Oral Biol Craniofac Res. 2018 Jan-Apr;8(1):48-53. doi: 10.1016/j.jobcr.2017.10.003. Epub 2017 Oct 19. J Oral Biol Craniofac Res. 2018. PMID: 29556464 Free PMC article. Review.
Cited by
-
Musculoskeletal Biomaterials: Stimulated and Synergized with Low Intensity Pulsed Ultrasound.J Funct Biomater. 2023 Oct 9;14(10):504. doi: 10.3390/jfb14100504. J Funct Biomater. 2023. PMID: 37888169 Free PMC article. Review.
-
Enhanced Vascularity in Gelatin Scaffolds via Copper-Doped Magnesium-Calcium Silicates Incorporation: In-Vitro and Ex-Ovo Insights.Ceram Int. 2024 Oct 15;50(20 Pt B):39889-39897. doi: 10.1016/j.ceramint.2024.07.369. Epub 2024 Jul 26. Ceram Int. 2024. PMID: 39618431
-
Stem Cells in Bone Tissue Engineering: Progress, Promises and Challenges.Stem Cell Rev Rep. 2024 Oct;20(7):1692-1731. doi: 10.1007/s12015-024-10738-y. Epub 2024 Jul 19. Stem Cell Rev Rep. 2024. PMID: 39028416 Review.
-
3D Printing of Hierarchically Porous Lattice Structures Based on Åkermanite Glass Microspheres and Reactive Silicone Binder.J Funct Biomater. 2022 Jan 13;13(1):8. doi: 10.3390/jfb13010008. J Funct Biomater. 2022. PMID: 35076529 Free PMC article.
-
Mesenchymal stem cells in craniofacial reconstruction: a comprehensive review.Front Mol Biosci. 2024 Apr 16;11:1362338. doi: 10.3389/fmolb.2024.1362338. eCollection 2024. Front Mol Biosci. 2024. PMID: 38690295 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

