Intracerebral Infection with E. coli Impairs Spatial Learning and Induces Necrosis of Hippocampal Neurons in the Tg2576 Mouse Model of Alzheimer's Disease
- PMID: 35530117
- PMCID: PMC9028720
- DOI: 10.3233/ADR-210049
Intracerebral Infection with E. coli Impairs Spatial Learning and Induces Necrosis of Hippocampal Neurons in the Tg2576 Mouse Model of Alzheimer's Disease
Abstract
Background: In patients with Alzheimer's disease (AD), bacterial infections are often associated with a cognitive decline. Animal models of genuine acute infections with viable bacteria which induce deterioration of neurodegenerative diseases are missing.
Objective: We assessed the effect of an intracerebral infection with E. coli in a mouse model of AD.
Methods: 13-month-old Tg2576 +/- mice and transgene negative littermates (Tg2576 -/-) received an intracerebral injection with E. coli K1 or saline followed by treatment with ceftriaxone starting 41 h post infection (p.i.) for 5 days. For 4 weeks, mice were monitored for clinical status, weight, motor functions, and neuropsychological status using the Morris water maze. ELISAs, stainings, and immunohistochemistry in brains were performed at the end of the experiment.
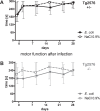
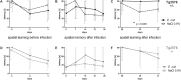
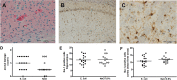
Results: Mortality of the infection was approximately 20%. After 4 weeks, spatial learning of infected Tg2576 +/- mice was compromised compared to non-infected Tg2576 +/- mice (p < 0.05). E. coli infection did not influence spatial learning in Tg2576 -/- mice, or spatial memory in both Tg2576 +/- and -/- mice within 4 weeks p.i.. Necrosis of hippocampal neurons was induced in infected compared to non-infected Tg2576 +/- mice 4 weeks p.i., whereas brain concentrations of Aβ1-40, Aβ1-42, and phosphoTau as well as axonal damage and microglia density were not altered.
Conclusion: Here, we proved in principle that a genuine acute bacterial infection can worsen cognitive functions of AD mice. Mouse models of subacute systemic infections are needed to develop new strategies for the treatment of bacterial infections in patients with AD in order to minimize their cognitive decline.
Keywords: Alzheimer’s disease; E. coli; amyloid-β; cognitive functions; delirium; intracerebral infection; neurodegenerative disease; spatial learning; spatial memory; water maze.
© 2022 – The authors. Published by IOS Press.
Conflict of interest statement
The authors have no conflict of interest to report.
Figures

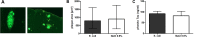

Similar articles
-
Recurrent systemic infections with Streptococcus pneumoniae do not aggravate the course of experimental neurodegenerative diseases.J Neurosci Res. 2010 Apr;88(5):1124-36. doi: 10.1002/jnr.22270. J Neurosci Res. 2010. PMID: 19859962
-
Impaired hippocampal acetylcholine release parallels spatial memory deficits in Tg2576 mice subjected to basal forebrain cholinergic degeneration.Brain Res. 2014 Jan 16;1543:253-62. doi: 10.1016/j.brainres.2013.10.055. Epub 2013 Nov 11. Brain Res. 2014. PMID: 24231553
-
Prevention of age-related spatial memory deficits in a transgenic mouse model of Alzheimer's disease by chronic Ginkgo biloba treatment.Exp Neurol. 2003 Nov;184(1):510-20. doi: 10.1016/s0014-4886(03)00399-6. Exp Neurol. 2003. PMID: 14637120
-
Using mice to model Alzheimer's dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models.Front Genet. 2014 Apr 23;5:88. doi: 10.3389/fgene.2014.00088. eCollection 2014. Front Genet. 2014. PMID: 24795750 Free PMC article. Review.
-
Pharmacological and molecular enhancement of learning in aging and Alzheimer's disease.J Physiol Paris. 2006 Mar-May;99(2-3):180-92. doi: 10.1016/j.jphysparis.2005.12.079. Epub 2006 Feb 3. J Physiol Paris. 2006. PMID: 16458491 Review.
Cited by
-
Alzheimer's-specific brain amyloid interactome: Neural-network analysis of intra-aggregate crosslinking identifies novel drug targets.iScience. 2023 Dec 18;27(1):108745. doi: 10.1016/j.isci.2023.108745. eCollection 2024 Jan 19. iScience. 2023. PMID: 38274404 Free PMC article.
-
Oh my gut! Is the microbial origin of neurodegenerative diseases real?Infect Immun. 2023 Oct 17;91(10):e0043722. doi: 10.1128/iai.00437-22. Epub 2023 Sep 26. Infect Immun. 2023. PMID: 37750713 Free PMC article. Review.
References
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–1858. - PMC - PubMed
-
- Perry VH, Cunningham C, Holmes C (2007) Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol 7, 161–167. - PubMed
LinkOut - more resources
Full Text Sources

