Engineering a folic acid-decorated ultrasmall gemcitabine nanocarrier for breast cancer therapy: Dual targeting of tumor cells and tumor-associated macrophages
- PMID: 35530140
- PMCID: PMC9072252
- DOI: 10.1016/j.apsb.2021.09.024
Engineering a folic acid-decorated ultrasmall gemcitabine nanocarrier for breast cancer therapy: Dual targeting of tumor cells and tumor-associated macrophages
Abstract
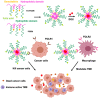
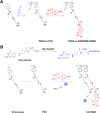
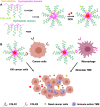
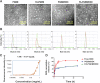
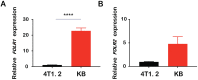
Combination of passive targeting with active targeting is a promising approach to improve the therapeutic efficacy of nanotherapy. However, most reported polymeric systems have sizes above 100 nm, which limits effective extravasation into tumors that are poorly vascularized and have dense stroma. This will, in turn, limit the overall effectiveness of the subsequent uptake by tumor cells via active targeting. In this study, we combined the passive targeting via ultra-small-sized gemcitabine (GEM)-based nanoparticles (NPs) with the active targeting provided by folic acid (FA) conjugation for enhanced dual targeted delivery to tumor cells and tumor-associated macrophages (TAMs). We developed an FA-modified prodrug carrier based on GEM (PGEM) to load doxorubicin (DOX), for co-delivery of GEM and DOX to tumors. The co-delivery system showed small particle size of ∼10 nm in diameter. The ligand-free and FA-targeted micelles showed comparable drug loading efficiency and a sustained DOX release profile. The FA-conjugated micelles effectively increased DOX uptake in cultured KB cancer cells that express a high level of folate receptor (FR), but no obvious increase was observed in 4T1.2 breast cancer cells that have a low-level expression of FR. Interestingly, in vivo, systemic delivery of FA-PGEM/DOX led to enhanced accumulation of the NPs in tumor and drastic reduction of tumor growth in a murine 4T1.2 breast cancer model. Mechanistic study showed that 4T1.2 tumor grown in mice expressed a significantly higher level of FOLR2, which was selectively expressed on TAMs. Thus, targeting of TAM may also contribute to the improved in vivo targeted delivery and therapeutic efficacy.
Keywords: Breast cancer; Doxorubicin; Dual targeting; Folic acid; Gemcitabine; Polymeric micelles; Tumor associated macrophages; Ultrasmall nanocarrier.
© 2022 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures
References
-
- Cabral H., Matsumoto Y., Mizuno K., Chen Q., Murakami M., Kimura M., et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6:815. - PubMed
-
- Hrkach J., Von Hoff D., Ali M.M., Andrianova E., Auer J., Campbell T., et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4 128ra39. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

