Microglia-Mediated Inflammation and Neural Stem Cell Differentiation in Alzheimer's Disease: Possible Therapeutic Role of KV1.3 Channel Blockade
- PMID: 35530176
- PMCID: PMC9070300
- DOI: 10.3389/fncel.2022.868842
Microglia-Mediated Inflammation and Neural Stem Cell Differentiation in Alzheimer's Disease: Possible Therapeutic Role of KV1.3 Channel Blockade
Abstract
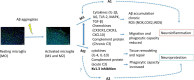
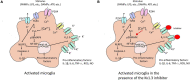
Increase of deposits of amyloid β peptides in the extracellular matrix is landmark during Alzheimer's Disease (AD) due to the imbalance in the production vs. clearance. This accumulation of amyloid β deposits triggers microglial activation. Microglia plays a dual role in AD, a protective role by clearing the deposits of amyloid β peptides increasing the phagocytic response (CD163, IGF-1 or BDNF) and a cytotoxic role, releasing free radicals (ROS or NO) and proinflammatory cytokines (TNF-α, IL-1β) in response to reactive gliosis activated by the amyloid β aggregates. Microglia activation correlated with an increase KV1.3 channels expression, protein levels and current density. Several studies highlight the importance of KV1.3 in the activation of inflammatory response and inhibition of neural progenitor cell proliferation and neuronal differentiation. However, little is known about the pathways of this activation in neural stem cells differentiation and proliferation and the role in amyloid β accumulation. In recent studies using in vitro cells derived from mice models, it has been demonstrated that KV1.3 blockers inhibit microglia-mediated neurotoxicity in culture reducing the expression and production of the pro-inflammatory cytokines IL-1β and TNF-α through the NF-kB and p38MAPK pathway. Overall, we conclude that KV1.3 blockers change the course of AD development, reducing microglial cytotoxic activation and increasing neural stem cell differentiation. However, further investigations are needed to establish the specific pathway and to validate the use of this blocker as therapeutic treatment in Alzheimer patients.
Keywords: Alzheimer’s disease; KV1.3; inflammation; microglia; neural stem cell (NSC); neurodegenaration; therapeutic targets.
Copyright © 2022 Revuelta, Urrutia, Villarroel and Casis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

