Stepwise engineering of Saccharomyces cerevisiae to produce (+)-valencene and its related sesquiterpenes
- PMID: 35530214
- PMCID: PMC9072130
- DOI: 10.1039/c9ra05558d
Stepwise engineering of Saccharomyces cerevisiae to produce (+)-valencene and its related sesquiterpenes
Abstract
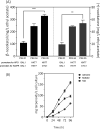
(+)-Valencene and (+)-nootkatone are high value-added sesquiterpenoids found in grapefruit. The synthesis of (+)-nootkatone by chemical oxidation from (+)-valencene cannot meet the increasing demand in natural aromatics markets. Development of a viable bioprocess using microorganisms is attractive. According to the yields of β-nootkatol and (+)-nootkatone by strains harboring different expression cassettes in the resting cell assay, premnaspirodiene oxygenase from Hyoscyamus muticus (HPO), cytochrome P450 reductase from Arabidopsis thaliana (AtCPR) and alcohol dehydrogenase (ADH1) from Saccharomyces cerevisiae were finally selected and overexpressed in CEN·PK2-1Ca, yielding β-nootkatol and (+)-nootkatone with 170.5 and 45.6 mg L-1 ethyl acetate, respectively. A combinational engineering strategy including promoter change, regulator ROX1 knockout, squalene pathway inhibition, and tHMGR overexpression was performed to achieve de novo (+)-valencene production. Subsequent culture investigations found that galactose as the induced carbon source and a lower temperature (25 °C) were beneficial to target accumulation. Also, replacing the inducible promoters (GAL1) of HPO and AtCPR with constitutive promoters (HXT7 and CYC1) dramatically increased the β-nootkatol accumulation from 108.2 to 327.8 mg L-1 ethyl acetate in resting-cell experiments using (+)-valencene as a substrate. Finally, the total terpenoid titer of the engineered strain of PK2-25 using glucose as a carbon source was improved to 157.8 mg L-1 cell culture, which was 56 times the initial value. We present a new candidate for production of (+)-valencene and its related sesquiterpenoids with attraction for industry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures






Similar articles
-
Metabolic engineering Saccharomyces cerevisiae for de novo production of the sesquiterpenoid (+)-nootkatone.Microb Cell Fact. 2020 Feb 3;19(1):21. doi: 10.1186/s12934-020-1295-6. Microb Cell Fact. 2020. PMID: 32013959 Free PMC article.
-
Production of the sesquiterpenoid (+)-nootkatone by metabolic engineering of Pichia pastoris.Metab Eng. 2014 Jul;24:18-29. doi: 10.1016/j.ymben.2014.04.001. Epub 2014 Apr 16. Metab Eng. 2014. PMID: 24747046
-
Probing the Synergistic Ratio of P450/CPR To Improve (+)-Nootkatone Production in Saccharomyces cerevisiae.J Agric Food Chem. 2022 Jan 26;70(3):815-825. doi: 10.1021/acs.jafc.1c07035. Epub 2022 Jan 11. J Agric Food Chem. 2022. PMID: 35015539
-
Production, Function, and Applications of the Sesquiterpenes Valencene and Nootkatone: a Comprehensive Review.J Agric Food Chem. 2023 Jan 11;71(1):121-142. doi: 10.1021/acs.jafc.2c07543. Epub 2022 Dec 21. J Agric Food Chem. 2023. PMID: 36541855 Review.
-
Nootkatone--a biotechnological challenge.Appl Microbiol Biotechnol. 2009 May;83(1):35-41. doi: 10.1007/s00253-009-1968-x. Epub 2009 Mar 31. Appl Microbiol Biotechnol. 2009. PMID: 19333595 Review.
Cited by
-
Compartmentalized Sesquiterpenoid Biosynthesis and Functionalization in the Chlamydomonas reinhardtii Plastid.Chembiochem. 2025 Feb 3;26(5):e202400902. doi: 10.1002/cbic.202400902. Epub 2024 Dec 5. Chembiochem. 2025. PMID: 39589357 Free PMC article.
-
Fermentation Strategies for Production of Pharmaceutical Terpenoids in Engineered Yeast.Pharmaceuticals (Basel). 2021 Mar 26;14(4):295. doi: 10.3390/ph14040295. Pharmaceuticals (Basel). 2021. PMID: 33810302 Free PMC article. Review.
-
Effect of Huanglongbing on the Volatile Organic Compound Profile of Fruit Juice and Peel Oil in 'Ray Ruby' Grapefruit.Foods. 2023 Feb 7;12(4):713. doi: 10.3390/foods12040713. Foods. 2023. PMID: 36832788 Free PMC article.
-
Focus and Insights into the Synthetic Biology-Mediated Chassis of Economically Important Fungi for the Production of High-Value Metabolites.Microorganisms. 2023 Apr 27;11(5):1141. doi: 10.3390/microorganisms11051141. Microorganisms. 2023. PMID: 37317115 Free PMC article. Review.
-
Advances on (+)-nootkatone microbial biosynthesis and its related enzymes.J Ind Microbiol Biotechnol. 2021 Aug 24;48(7-8):kuab046. doi: 10.1093/jimb/kuab046. J Ind Microbiol Biotechnol. 2021. PMID: 34279658 Free PMC article.
References
-
- Haring H. G. Rijkens F. Boelens H. Van der Gen A. J. Agric. Food Chem. 1972;20:1018–1021. doi: 10.1021/jf60183a011. - DOI
-
- FEEDAP EFSA J. 2016;14:4475.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

