Oxone promoted dehydrogenative Povarov cyclization of N-aryl glycine derivatives: an approach towards quinoline fused lactones and lactams
- PMID: 35530246
- PMCID: PMC9072217
- DOI: 10.1039/c9ra06212b
Oxone promoted dehydrogenative Povarov cyclization of N-aryl glycine derivatives: an approach towards quinoline fused lactones and lactams
Abstract
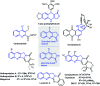
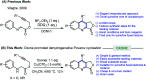
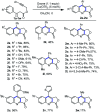
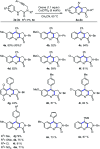
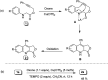
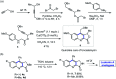
Oxone promoted intramolecular dehydrogenative imino Diels-Alder reaction (Povarov cyclization) of alkyne tethered N-aryl glycine esters and amides has been explored, thus affording biologically significant quinoline fused lactones and lactams. The reaction is simple, scalable, and high yielding (up to 88%). The method was further extended to prepare biologically important luotonin-A analogues and the quinoline core of uncialamycin.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
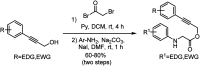
References
-
- Sharma R. Kour P. Kumar A. J. Chem. Sci. 2018;130:73. doi: 10.1007/s12039-018-1466-8. - DOI
- Solomon V. R. Lee H. Curr. Med. Chem. 2011;18:1488–1508. doi: 10.2174/092986711795328382. - DOI - PubMed
- Musiol R. Serda M. Hensel-Bielowka S. Polanski J. Curr. Med. Chem. 2010;17:1960. doi: 10.2174/092986710791163966. - DOI - PubMed
- Musiol R. Jampilek J. Kralova K. Richardson D. R. Kalinowski D. Podeszwa B. Finster J. Niedbala H. Palka A. Polanski J. Bioorg. Med. Chem. 2007;15:1280–1288. doi: 10.1016/j.bmc.2006.11.020. - DOI - PubMed
- Michael J. P. Nat. Prod. Rep. 2007;24:223–246. doi: 10.1039/B509528J. - DOI - PubMed
- Kouznetsov V. V. Mendez L. Y. V. Gomez C. M. M. Curr. Org. Chem. 2005;9:141–161. doi: 10.2174/1385272053369196. - DOI
-
- Boisse T. Gavara L. Gautret P. Baldeyrou B. Lansiaux A. Goossens J.-F. Hénichart J.-P. Rigo B. Tetrahedron Lett. 2011;52:1592–1596. doi: 10.1016/j.tetlet.2011.01.105. - DOI
- Jahng K. C. Kim S. I. Kim D. H. Seo C. S. Son J.-K. Lee S. H. Lee E. S. Jahng Y. Chem. Pharm. Bull. 2008;56:607–609. doi: 10.1248/cpb.56.607. - DOI - PubMed
- Cagir A. Eisenhauer B. M. Gao R. Thomas S. J. Hecht S. M. Bioorg. Med. Chem. 2004;12:6287–6299. doi: 10.1016/j.bmc.2004.08.052. - DOI - PubMed
- Lee E. S. Park J.-G. Jahng Y. Tetrahedron Lett. 2003;44:1883–1886. doi: 10.1016/S0040-4039(03)00080-7. - DOI
- Dallavalle S. Merlini L. Tetrahedron Lett. 2002;43:1835–1837. doi: 10.1016/S0040-4039(02)00140-5. - DOI
- Yadav J. S. Reddy B. V. S. Tetrahedron Lett. 2002;43:1905–1907. doi: 10.1016/S0040-4039(02)00135-1. - DOI
- Wang H. Ganesan A. Tetrahedron Lett. 1998;39:9097–9098. doi: 10.1016/S0040-4039(98)02004-8. - DOI
- Tseng M.-C. Chu Y.-W. Tsai H.-P. Lin C.-M. Hwang J. Chu Y.-H. Org. Lett. 2011;13:920–923. doi: 10.1021/ol1029707. - DOI - PubMed
- Kwon S. H. Seo H.-A. Cheon C.-H. Org. Lett. 2016;18:5280–5283. doi: 10.1021/acs.orglett.6b02597. - DOI - PubMed
-
- Nicolaou K. C. Wang Y. Lu M. Mandal D. Pattanayak M. R. Yu R. Shah A. A. Chen J. S. Zhang H. Crawford J. J. Pasunoori L. Poudel Y. B. Chowdari N. S. Pan C. Nazeer A. Gangwar S. Vite G. Pitsinos E. N. J. Am. Chem. Soc. 2016;138:8235–8246. doi: 10.1021/jacs.6b04339. - DOI - PubMed
- Nicolaou K. C. Chen J. S. Zhang H. Montero A. Angew. Chem., Int. Ed. 2008;47:185–189. doi: 10.1002/anie.200704577. - DOI - PubMed
- Nicolaou K. C. Zhang H. Chen J. S. Crawford J. J. Pasunoori L. Angew. Chem., Int. Ed. 2007;46:4704–4707. doi: 10.1002/anie.200700917. - DOI - PubMed
-
- Chen Q. Zhang S. Zhang T. He K. Yuan Y. Jia X. Asian J. Org. Chem. 2019;8:115–118. doi: 10.1002/ajoc.201800596. - DOI
- Liu Y. Q. Yang L. Tian X. Curr. Bioact. Compd. 2007;3:37. doi: 10.2174/157340707780126499. - DOI
- Gordaliza M. Garcia P. A. Miguel del Corral J. M. Castro M. A. Gomez-Zurita M. A. Toxicon. 2004;44:441. doi: 10.1016/j.toxicon.2004.05.008. - DOI - PubMed
- Feliciano A. S. Miguel del Corral J. M. Gordaliza M. Castro M. A. Phytochemistry. 1989;28:659–660. doi: 10.1016/0031-9422(89)80081-0. - DOI
-
- Blair A. Zmuda F. Malviya G. Tavares A. A. S. Tamagnan G. D. Chalmers A. J. Dewar D. Pimlott S. L. Sutherland A. Chem. Sci. 2015;6:4772–4777. doi: 10.1039/C5SC01647A. - DOI - PMC - PubMed
- Blair A. Stevenson L. Dewar D. Pimlott S. L. Sutherland A. MedChemComm. 2013;4:1461–1466. doi: 10.1039/C3MD00249G. - DOI
- Stevenson L. Tavares A. A. S. Brunet A. McGonagle F. I. Dewar D. Pimlott S. L. Sutherland A. Bioorg. Med. Chem. Lett. 2010;20:954–957. doi: 10.1016/j.bmcl.2009.12.061. - DOI - PubMed
- Anzini M. Cappelli A. Vomero S. Seeber M. Menziani M. C. Langer T. Hagen B. Manzoni C. Bourguignon J.-J. J. Med. Chem. 2001;44:1134–1150. doi: 10.1021/jm0009742. - DOI - PubMed
LinkOut - more resources
Full Text Sources

