An insight into the intermolecular vibrational modes of dicationic ionic liquids through far-infrared spectroscopy and DFT calculations
- PMID: 35530250
- PMCID: PMC9072084
- DOI: 10.1039/c9ra05735h
An insight into the intermolecular vibrational modes of dicationic ionic liquids through far-infrared spectroscopy and DFT calculations
Abstract
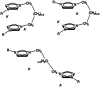
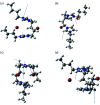
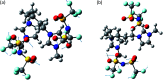
Dicationic ionic liquids (DILs) are a subclass of the ionic liquid (IL) family and are characterized by two cationic head groups linked by means of a spacer. While DILs are increasingly attracting interest due to their peculiar physico-chemical properties, there is still a lack of understanding of their intermolecular interactions. Herein, we report our investigations on the intermolecular vibrational modes of two bromide DILs and of a bistriflimide DIL. The minimal possible neutral cluster of ions was studied as a simplified model of these systems and was optimized at the DFT level. Normal modes of two sandwich-like conformers were then calculated using the harmonic approximation with analytical computation of the second derivatives of molecular energy with respect to the atomic coordinates. The calculated spectra were compared to far-infrared experimental spectra and two groups of peaks over three, for the two bromide DILs, and three over five, for the Tf2N- DIL, were described by the proposed neutral cluster model. Therefore, this model represents a reliable and computationally affordable model for the exploration of the intermolecular interactions of this kind of system.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures
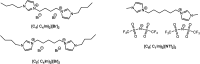


Similar articles
-
DFT and COSMO-RS studies on dicationic ionic liquids (DILs) as potential candidates for CO2 capture: the effects of alkyl side chain length and symmetry in cations.RSC Adv. 2022 Dec 9;12(54):35418-35435. doi: 10.1039/d2ra05805g. eCollection 2022 Dec 6. RSC Adv. 2022. PMID: 36540232 Free PMC article.
-
Systematic Synthesis and Properties Evaluation of Dicationic Ionic Liquids, and a Glance Into a Potential New Field.Front Chem. 2018 Dec 12;6:612. doi: 10.3389/fchem.2018.00612. eCollection 2018. Front Chem. 2018. PMID: 30619821 Free PMC article.
-
CO2 capture using dicationic ionic liquids (DILs): Molecular dynamics and DFT-IR studies on the role of cations.J Chem Phys. 2023 Jan 14;158(2):024503. doi: 10.1063/5.0131507. J Chem Phys. 2023. PMID: 36641394
-
Recent Advances in Imidazolium-Based Dicationic Ionic Liquids as Organocatalysts: A Mini-Review.Materials (Basel). 2022 Jan 23;15(3):866. doi: 10.3390/ma15030866. Materials (Basel). 2022. PMID: 35160810 Free PMC article. Review.
-
Two-dimensional infrared spectroscopy of intermolecular hydrogen bonds in the condensed phase.Acc Chem Res. 2009 Sep 15;42(9):1220-8. doi: 10.1021/ar900006u. Acc Chem Res. 2009. PMID: 19425543 Review.
Cited by
-
Effect of Bulky Anion around the Dication on the Electronic Structure and Normal Frequencies in 1,3-Bis(3-methylimidazolium-1-yl)propane Bis(trifluoromethanesulfonyl)imide Ionic Liquid.ACS Omega. 2021 Sep 1;6(36):23293-23299. doi: 10.1021/acsomega.1c03017. eCollection 2021 Sep 14. ACS Omega. 2021. PMID: 34549129 Free PMC article.
-
A Specific Interaction between Ionic Liquids' Cations and Reichardt's Dye.Molecules. 2022 Oct 25;27(21):7205. doi: 10.3390/molecules27217205. Molecules. 2022. PMID: 36364035 Free PMC article.
-
DFT and COSMO-RS studies on dicationic ionic liquids (DILs) as potential candidates for CO2 capture: the effects of alkyl side chain length and symmetry in cations.RSC Adv. 2022 Dec 9;12(54):35418-35435. doi: 10.1039/d2ra05805g. eCollection 2022 Dec 6. RSC Adv. 2022. PMID: 36540232 Free PMC article.
References
-
- Cao Y. Mu T. Ind. Eng. Chem. Res. 2014;53:8651–8664. doi: 10.1021/ie5009597. - DOI
-
- Fox D. M. Gilman J. W. Morgan A. B. Shields J. R. Maupin P. H. Lyon R. E. De Long H. C. Trulove P. C. Ind. Eng. Chem. Res. 2008;47:6327–6332. doi: 10.1021/ie800665u. - DOI
-
- Seddon K. Chem. Eng. 2002;730:33–35.
-
- Silva S. S. Mano J. F. Reis R. L. Green Chem. 2017;19:1208–1220. doi: 10.1039/C6GC02827F. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

