Ivermectin represses Wnt/β-catenin signaling by binding to TELO2, a regulator of phosphatidylinositol 3-kinase-related kinases
- PMID: 35530256
- PMCID: PMC9072907
- DOI: 10.1016/j.isci.2022.103912
Ivermectin represses Wnt/β-catenin signaling by binding to TELO2, a regulator of phosphatidylinositol 3-kinase-related kinases
Abstract
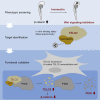
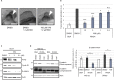
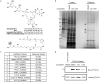
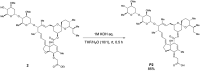
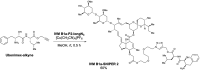
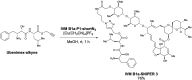
Ivermectin (IVM), an avermectin-derivative anthelmintic, specifically binds to glutamate-gated chloride ion channels (GluCls), causing paralysis in invertebrates. IVM also exhibits other biological activities such as Wnt/β-catenin pathway inhibition in vertebrates that do not possess GluCls. This study showed that affinity purification using immobilized IVM B1a isolated TELO2, a cofactor of phosphatidylinositol 3-kinase-related kinases (PIKKs), as a specific IVM B1a-binding protein. TELO2 knockdown reduced cytoplasmic β-catenin and the transcriptional activation of β-catenin/TCF. IVM B1a bound to TELO2 through the C-terminal α-helix, in which mutations conferred IVM resistance. IVM reduced the TELO2 and PIKK protein levels and the AKT and S6 kinase phosphorylation levels. The inhibition of mTOR kinase reduced the cytoplasmic β-catenin level. Therefore, IVM binds to TELO2, inhibiting PIKKs and reducing the cytoplasmic β-catenin level. In conclusion, our data indicate TELO2 as a druggable target for human diseases involving abnormalities of the Wnt/β-catenin pathway and PIKKs, including mTOR.
Keywords: Biochemistry; Molecular biology; Small molecule.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures







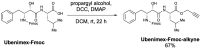
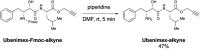

Similar articles
-
Synthesis of photoreactive ivermectin B1a derivatives and their actions on Haemonchus and Bombyx glutamate-gated chloride channels.Pestic Biochem Physiol. 2015 May;120:82-90. doi: 10.1016/j.pestbp.2014.10.017. Epub 2014 Nov 4. Pestic Biochem Physiol. 2015. PMID: 25987225
-
Structural mechanism underlying the differential effects of ivermectin and moxidectin on the C. elegans glutamate-gated chloride channel GLC-2.Biomed Pharmacother. 2022 Jan;145:112380. doi: 10.1016/j.biopha.2021.112380. Epub 2021 Nov 5. Biomed Pharmacother. 2022. PMID: 34749053
-
Ivermectin activates GIRK channels in a PIP2 -dependent, Gβγ -independent manner and an amino acid residue at the slide helix governs the activation.J Physiol. 2017 Sep 1;595(17):5895-5912. doi: 10.1113/JP274871. Epub 2017 Jul 30. J Physiol. 2017. PMID: 28715108 Free PMC article.
-
New steps in the Wnt/beta-catenin signal transduction pathway.Recent Prog Horm Res. 2000;55:225-36. Recent Prog Horm Res. 2000. PMID: 11036939 Review.
-
Dysregulation of Wnt/β-catenin signaling by protein kinases in hepatocellular carcinoma and its therapeutic application.Cancer Sci. 2021 May;112(5):1695-1706. doi: 10.1111/cas.14861. Epub 2021 Apr 6. Cancer Sci. 2021. PMID: 33605517 Free PMC article. Review.
Cited by
-
TTT (Tel2-Tti1-Tti2) Complex, the Co-Chaperone of PIKKs and a Potential Target for Cancer Chemotherapy.Int J Mol Sci. 2023 May 5;24(9):8268. doi: 10.3390/ijms24098268. Int J Mol Sci. 2023. PMID: 37175973 Free PMC article. Review.
-
CD58 acts as a tumor promotor in hepatocellular carcinoma via activating the AKT/GSK-3β/β-catenin pathway.J Transl Med. 2023 Aug 12;21(1):539. doi: 10.1186/s12967-023-04364-4. J Transl Med. 2023. PMID: 37573318 Free PMC article.
-
Computational Modeling to Identify Drugs Targeting Metastatic Castration-Resistant Prostate Cancer Characterized by Heightened Glycolysis.Pharmaceuticals (Basel). 2024 Apr 29;17(5):569. doi: 10.3390/ph17050569. Pharmaceuticals (Basel). 2024. PMID: 38794139 Free PMC article.
-
Isovalerylspiramycin I Reprograms the Immunosuppressive and Temozolomide-Resistant Microenvironment by Inhibiting the Frizzled-5/Wnt/β-Catenin Pathway in Glioblastoma.Research (Wash D C). 2025 Aug 13;8:0828. doi: 10.34133/research.0828. eCollection 2025. Research (Wash D C). 2025. PMID: 40809457 Free PMC article.
-
Exploring the Functional Roles of Telomere Maintenance 2 in the Tumorigenesis of Glioblastoma Multiforme and Drug Responsiveness to Temozolomide.Int J Mol Sci. 2023 May 25;24(11):9256. doi: 10.3390/ijms24119256. Int J Mol Sci. 2023. PMID: 37298208 Free PMC article.
References
-
- Arena J.P., Liu K.K., Paress P.S., Schaeffer J.M., Cully D.F. Expression of a glutamate-activated chloride current in Xenopus oocytes injected with Caenorhabditis elegans RNA: evidence for modulation by avermectin. Brain Res. Mol. Brain Res. 1992;15:339–348. doi: 10.1016/0169-328x(92)90127-w. - DOI - PubMed
-
- Burg R.W., Miller B.M., Baker E.E., Birnbaum J., Currie S.A., Hartman R., Kong Y.L., Monaghan R.L., Olson G., Putter I., et al. Avermectins, new family of potent anthelmintic agents: producing organism and fermentation. Antimicrob. Agents Chemother. 1979;15:361–367. doi: 10.1128/AAC.15.3.361. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

