Pyroptosis impacts the prognosis and treatment response in gastric cancer via immune system modulation
- PMID: 35530274
- PMCID: PMC9077078
Pyroptosis impacts the prognosis and treatment response in gastric cancer via immune system modulation
Abstract
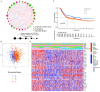
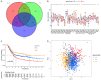
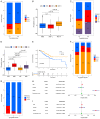
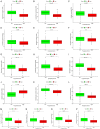
Pyroptosis plays a vital role in the development of cancers; however, its role in regulating immune cell infiltration in tumor microenvironment (TME) and pyroptosis-related molecular subtypes remain unclear. Herein, we comprehensively analyzed the molecular subtypes mediated by the pyroptosis-related genes (PRGs) in gastric cancer (GC). Three pyroptosis patterns were determined with distinct TME cell-infiltrating characteristics and prognosis. Principal component analysis was performed to establish the pyroptosis score. The high pyroptosis score group was featured by increased activated CD4+ T cell infiltration, better prognosis, elevated tumor mutation burden, higher immune and stromal scores, and enhanced response to immunotherapy. However, the low pyroptosis score group was characterized by poorer survival, decreased immune infiltration, and glycerolipid and histidine metabolism pathways. Additionally, high pyroptosis score was confirmed as an independent favorable prognostic factor for overall survival. Three cohorts designed to analyze the response to immunotherapy verified that patients with higher pyroptosis score showed treatment benefit. In summary, our study demonstrated that pyroptosis regulates the complex TME. Assessing the pyroptosis patterns will advance our understanding on TME features and tumor immunology and provide the rationale for designing personalized immunotherapy strategies.
Keywords: Pyroptosis; gastric cancer; immunotherapy; prognosis; tumor microenvironment.
AJCR Copyright © 2022.
Conflict of interest statement
None.
Figures



References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. - PubMed
-
- Yang W, Ma J, Zhou W, Cao B, Zhou X, Yang Z, Zhang H, Zhao Q, Fan D, Hong L. Molecular mechanisms and theranostic potential of miRNAs in drug resistance of gastric cancer. Expert Opin Ther Targets. 2017;21:1063–1075. - PubMed
-
- Johnston FM, Beckman M. Updates on management of gastric cancer. Curr Oncol Rep. 2019;21:67. - PubMed
-
- Helmy KY, Patel SA, Nahas GR, Rameshwar P. Cancer immunotherapy: accomplishments to date and future promise. Ther Deliv. 2013;4:1307–1320. - PubMed
-
- Zhuo M, Chi Y, Wang Z. The adverse events associated with combination immunotherapy in cancers: challenges and chances. Asia Pac J Clin Oncol. 2020;16:e154–e159. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
