The small molecule NLRP3 inhibitor RRx-001 potentiates regorafenib activity and attenuates regorafenib-induced toxicity in mice bearing human colorectal cancer xenografts
- PMID: 35530283
- PMCID: PMC9077073
The small molecule NLRP3 inhibitor RRx-001 potentiates regorafenib activity and attenuates regorafenib-induced toxicity in mice bearing human colorectal cancer xenografts
Abstract
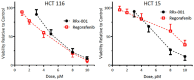
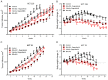
The multi-kinase inhibitor Regorafenib, approved for the treatment of metastatic colorectal cancer, is poorly tolerated with a Grade 3/4 drug related adverse event rate of 54% resulting in frequent dose reductions and discontinuations. RRx-001 is a minimally toxic NLRP3 inhibitor small molecule with macrophage-repolarizing properties in Phase 3 clinical trials. Studies have demonstrated the inhibitory impact of M2 macrophages on the activity of tyrosine kinases, suggesting that the repolarization of macrophages by RRx-001 may enhance the activity of TKIs. The purpose of these experiments was to determine whether RRx-001 demonstrated in vitro and in vivo synergy with regorafenib in colorectal cancer and whether RRx-001 attenuated the toxicity of regorafenib. Tumor-bearing mice were randomized into four cohorts: RRx-001 alone, regorafenib alone, RRx-001 + regorafenib and control. RRx-001 demonstrated in vitro and in vivo synergy with regorafenib with attenuation of toxicity in colorectal cancer cell lines. These results provide a rationale to treat colorectal cancer with RRx-001 plus another tyrosine kinase inhibitor like regorafenib.
Keywords: Chemotherapy; RRx-001; colorectal cancer; regorafenib; tyrosine kinase inhibitor.
AJCR Copyright © 2022.
Conflict of interest statement
None.
Figures
References
-
- Rosen SA, Buell JF, Yoshida A, Kazsuba S, Hurst R, Michelassi F, Millis JM, Posner MC. Initial presentation with stage IV colorectal cancer how aggressive should we be? Arch Surg. 2000;135:530–534. - PubMed
