Targeting 2-oxoglutarate dehydrogenase for cancer treatment
- PMID: 35530286
- PMCID: PMC9077069
Targeting 2-oxoglutarate dehydrogenase for cancer treatment
Abstract
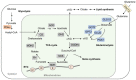
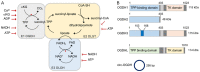
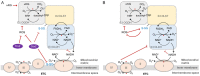
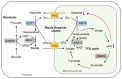
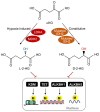
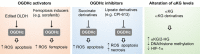
Tricarboxylic acid (TCA) cycle, also called Krebs cycle or citric acid cycle, is an amphoteric pathway, contributing to catabolic degradation and anaplerotic reactions to supply precursors for macromolecule biosynthesis. Oxoglutarate dehydrogenase complex (OGDHc, also called α-ketoglutarate dehydrogenase) a highly regulated enzyme in TCA cycle, converts α-ketoglutarate (αKG) to succinyl-Coenzyme A in accompany with NADH generation for ATP generation through oxidative phosphorylation. The step collaborates with glutaminolysis at an intersectional point to govern αKG levels for energy production, nucleotide and amino acid syntheses, and the resources for macromolecule synthesis in cancer cells with rapid proliferation. Despite being a flavoenzyme susceptible to electron leakage contributing to mitochondrial reactive oxygen species (ROS) production, OGDHc is highly sensitive to peroxides such as HNE (4-hydroxy-2-nonenal) and moreover, its activity mediates the activation of several antioxidant pathways. The characteristics endow OGDHc as a critical redox sensor in mitochondria. Accumulating evidences suggest that dysregulation of OGDHc impairs cellular redox homeostasis and disturbs substrate fluxes, leading to a buildup of oncometabolites along the pathogenesis and development of cancers. In this review, we describe molecular interactions, regulation of OGDHc expression and activity and its relationships with diseases, specifically focusing on cancers. In the end, we discuss the potential of OGDHs as a therapeutic target for cancer treatment.
Keywords: 2-oxoglutarate dehydrogenase; cancer metabolism; reactive oxygen species; tricarboxylic acid cycle; α-ketoglutarate dehydrogenase complex.
AJCR Copyright © 2022.
Conflict of interest statement
None.
Figures
References
-
- Maas E, Bisswanger H. Localization of the alpha-oxoacid dehydrogenase multi-enzyme complexes within the mitochondrion. FEBS Lett. 1990;277:189–190. - PubMed
-
- Sumegi B, Srere PA. Complex I binds several mitochondrial NAD-coupled dehydrogenases. J Biol Chem. 1984;259:15040–15045. - PubMed
-
- Yang L, Venneti S, Nagrath D. Glutaminolysis: a hallmark of cancer metabolism. Annu Rev Biomed Eng. 2017;19:163–194. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
