DNA repair proteins as the targets for paroxetine to induce cytotoxicity in gastric cancer cell AGS
- PMID: 35530295
- PMCID: PMC9077064
DNA repair proteins as the targets for paroxetine to induce cytotoxicity in gastric cancer cell AGS
Abstract
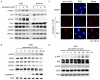
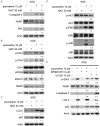
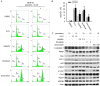
To evaluate the potential anticancer effects of 1175 FDA-approved drugs, cell viability screening was performed using 25 human cancer cell lines covering 14 human cancer types. Here, we focus on the action of paroxetine, which demonstrated greater toxicity toward human gastric adenocarcinoma cell-line AGS cells compared with the other FDA-approved drugs, exhibiting an IC50 value lower than 10 μM. Evaluation of the underlying novel mechanisms revealed that paroxetine can enhance DNA damage in gastric cancer cells and involves downregulation of Rad51, HR23B and ERCC1 expression and function, as well as nucleotide shortage. Enhancement of autophagy counteracted paroxetine-induced apoptosis but did not affect paroxetine-induced DNA damage. Paroxetine also enhanced ROS generation in AGS cells, but a ROS scavenger did not improve paroxetine-mediated DNA damage, apoptosis, or autophagy, suggesting ROS might play a minor role in paroxetine-induced cell toxicity. In contrast, paroxetine did not enhance DNA damage, apoptosis, or autophagy in another insensitive gastric adenocarcinoma cell-line MKN-45 cells. Interestingly, co-administration of paroxetine with conventional anticancer agents sensitized MKN-45 cells to these agents: co-treated cells showed increased apoptosis relative to MKN-45 cells treated with the anticancer agent alone. Unequivocally, these data suggest that for the first time that paroxetine triggers cytotoxicity and DNA damage in AGS cells at least partly by reducing the gene expression of Rad51, HR23B, and ERCC1. Our findings also suggest that paroxetine is a promising candidate anticancer agent and/or chemosensitizing agent for use in combination with other anticancer drugs in cancer therapy. The molecular mechanisms underlying the anticancer activity of co-treatment with paroxetine and chemotherapy appear to be complex and are worthy of further investigation.
Keywords: DNA damage; Gastric cancer; apoptosis; chemosensitizer; drug repurposing; paroxetine.
AJCR Copyright © 2022.
Conflict of interest statement
None.
Figures







Similar articles
-
Inhibition of Topoisomerase IIα and Induction of Apoptosis in Gastric Cancer Cells by 19-Triisopropyl Andrographolide.Asian Pac J Cancer Prev. 2017 Oct 26;18(10):2845-2851. doi: 10.22034/APJCP.2017.18.10.2845. Asian Pac J Cancer Prev. 2017. PMID: 29072435 Free PMC article.
-
TRIB3 as a biomarker of gastric cancer cell sensitivity to chemotherapeutic agents running title: A protective role of TRIB3 on chemotherapy.SAGE Open Med. 2024 Oct 30;12:20503121241292673. doi: 10.1177/20503121241292673. eCollection 2024. SAGE Open Med. 2024. PMID: 39483625 Free PMC article.
-
DNA damage, cell cycle perturbation and cell death by naphthalene diimide derivative in gastric cancer cells.Chem Biol Interact. 2022 May 1;358:109881. doi: 10.1016/j.cbi.2022.109881. Epub 2022 Mar 17. Chem Biol Interact. 2022. PMID: 35307378
-
Chalcone flavokawain B induces autophagic-cell death via reactive oxygen species-mediated signaling pathways in human gastric carcinoma and suppresses tumor growth in nude mice.Arch Toxicol. 2017 Oct;91(10):3341-3364. doi: 10.1007/s00204-017-1967-0. Epub 2017 Apr 3. Arch Toxicol. 2017. PMID: 28374157
-
Downregulation of c-Myc and p21 expression and induction of S phase arrest by naphthalene diimide derivative in gastric adenocarcinoma cells.Chem Biol Interact. 2019 May 1;304:106-123. doi: 10.1016/j.cbi.2019.02.010. Epub 2019 Mar 3. Chem Biol Interact. 2019. PMID: 30840857
Cited by
-
Inhibition of TNBC Cell Growth by Paroxetine: Induction of Apoptosis and Blockage of Autophagy Flux.Cancers (Basel). 2024 Feb 22;16(5):885. doi: 10.3390/cancers16050885. Cancers (Basel). 2024. PMID: 38473249 Free PMC article.
-
Purinergic signaling: Diverse effects and therapeutic potential in cancer.Front Oncol. 2023 Jan 18;13:1058371. doi: 10.3389/fonc.2023.1058371. eCollection 2023. Front Oncol. 2023. PMID: 36741002 Free PMC article. Review.
-
Antidepressants as Autophagy Modulators for Cancer Therapy.Molecules. 2023 Nov 14;28(22):7594. doi: 10.3390/molecules28227594. Molecules. 2023. PMID: 38005316 Free PMC article. Review.
-
Adapalene potentiates the cytotoxicity of anti-cancer drugs by enhancing cell cycle dysregulation and apoptosis in MKN-45 cells.Toxicol Res (Camb). 2025 Feb 12;14(1):tfaf017. doi: 10.1093/toxres/tfaf017. eCollection 2025 Feb. Toxicol Res (Camb). 2025. PMID: 39949368
-
Repurposed Drugs in Gastric Cancer.Molecules. 2022 Dec 30;28(1):319. doi: 10.3390/molecules28010319. Molecules. 2022. PMID: 36615513 Free PMC article. Review.
References
-
- Vendrely V, Peuchant E, Buscail E, Moranvillier I, Rousseau B, Bedel A, Brillac A, de Verneuil H, Moreau-Gaudry F, Dabernat S. Resveratrol and capsaicin used together as food complements reduce tumor growth and rescue full efficiency of low dose gemcitabine in a pancreatic cancer model. Cancer Lett. 2017;390:91–102. - PubMed
-
- Chou CT, He S, Jan CR. Paroxetine-induced apoptosis in human osteosarcoma cells: activation of p38 MAP kinase and caspase-3 pathways without involvement of [Ca2+]i elevation. Toxicol Appl Pharmacol. 2007;218:265–273. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
