Intrinsic Differences in Spatiotemporal Organization and Stromal Cell Interactions Between Isogenic Lung Cancer Cells of Epithelial and Mesenchymal Phenotypes Revealed by High-Dimensional Single-Cell Analysis of Heterotypic 3D Spheroid Models
- PMID: 35530312
- PMCID: PMC9076321
- DOI: 10.3389/fonc.2022.818437
Intrinsic Differences in Spatiotemporal Organization and Stromal Cell Interactions Between Isogenic Lung Cancer Cells of Epithelial and Mesenchymal Phenotypes Revealed by High-Dimensional Single-Cell Analysis of Heterotypic 3D Spheroid Models
Abstract
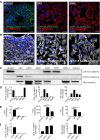
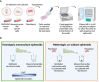
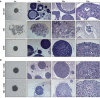
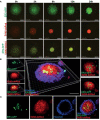
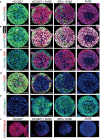
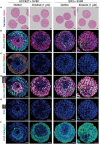
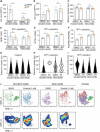
The lack of inadequate preclinical models remains a limitation for cancer drug development and is a primary contributor to anti-cancer drug failures in clinical trials. Heterotypic multicellular spheroids are three-dimensional (3D) spherical structures generated by self-assembly from aggregates of two or more cell types. Compared to traditional monolayer cell culture models, the organization of cells into a 3D tissue-like structure favors relevant physiological conditions with chemical and physical gradients as well as cell-cell and cell-extracellular matrix (ECM) interactions that recapitulate many of the hallmarks of cancer in situ. Epidermal growth factor receptor (EGFR) mutations are prevalent in non-small cell lung cancer (NSCLC), yet various mechanisms of acquired resistance, including epithelial-to-mesenchymal transition (EMT), limit the clinical benefit of EGFR tyrosine kinase inhibitors (EGFRi). Improved preclinical models that incorporate the complexity induced by epithelial-to-mesenchymal plasticity (EMP) are urgently needed to advance new therapeutics for clinical NSCLC management. This study was designed to provide a thorough characterization of multicellular spheroids of isogenic cancer cells of various phenotypes and demonstrate proof-of-principle for the applicability of the presented spheroid model to evaluate the impact of cancer cell phenotype in drug screening experiments through high-dimensional and spatially resolved imaging mass cytometry (IMC) analyses. First, we developed and characterized 3D homotypic and heterotypic spheroid models comprising EGFRi-sensitive or EGFRi-resistant NSCLC cells. We observed that the degree of EMT correlated with the spheroid generation efficiency in monocultures. In-depth characterization of the multicellular heterotypic spheroids using immunohistochemistry and high-dimensional single-cell analyses by IMC revealed intrinsic differences between epithelial and mesenchymal-like cancer cells with respect to self-sorting, spatiotemporal organization, and stromal cell interactions when co-cultured with fibroblasts. While the carcinoma cells harboring an epithelial phenotype self-organized into a barrier sheet surrounding the fibroblasts, mesenchymal-like carcinoma cells localized to the central hypoxic and collagen-rich areas of the compact heterotypic spheroids. Further, deep-learning-based single-cell segmentation of IMC images and application of dimensionality reduction algorithms allowed a detailed visualization and multiparametric analysis of marker expression across the different cell subsets. We observed a high level of heterogeneity in the expression of EMT markers in both the carcinoma cell populations and the fibroblasts. Our study supports further application of these models in pre-clinical drug testing combined with complementary high-dimensional single-cell analyses, which in turn can advance our understanding of the impact of cancer-stroma interactions and epithelial phenotypic plasticity on innate and acquired therapy resistance in NSCLC.
Keywords: drug resistance; erlotinib-resistance; heterotypic 3D models; imaging mass cytometry; in vitro cell culture models; non-small cell lung cancer (NSCLC); tumor microenvironment.
Copyright © 2022 Lotsberg, Røsland, Rayford, Dyrstad, Ekanger, Lu, Frantz, Stuhr, Ditzel, Thiery, Akslen, Lorens and Engelsen.
Conflict of interest statement
Author AR was employed by BerGenBio. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Characterization of 3D NSCLC Cell Cultures with Fibroblasts or Macrophages for Tumor Microenvironment Studies and Chemotherapy Screening.Cells. 2023 Dec 8;12(24):2790. doi: 10.3390/cells12242790. Cells. 2023. PMID: 38132110 Free PMC article.
-
In-air production of 3D co-culture tumor spheroid hydrogels for expedited drug screening.Acta Biomater. 2019 Aug;94:392-409. doi: 10.1016/j.actbio.2019.06.012. Epub 2019 Jun 12. Acta Biomater. 2019. PMID: 31200118
-
Effect of homotypic and heterotypic interaction in 3D on the E-selectin mediated adhesive properties of breast cancer cell lines.Biomaterials. 2012 Dec;33(35):9037-48. doi: 10.1016/j.biomaterials.2012.08.052. Epub 2012 Sep 17. Biomaterials. 2012. PMID: 22992472 Free PMC article.
-
Heterotypic tumor spheroids: a platform for nanomedicine evaluation.J Nanobiotechnology. 2023 Aug 2;21(1):249. doi: 10.1186/s12951-023-02021-y. J Nanobiotechnology. 2023. PMID: 37533100 Free PMC article. Review.
-
The role of epithelial to mesenchymal transition in resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer.Transl Lung Cancer Res. 2016 Apr;5(2):172-82. doi: 10.21037/tlcr.2016.04.07. Transl Lung Cancer Res. 2016. PMID: 27186512 Free PMC article. Review.
Cited by
-
AXL expression reflects tumor-immune cell dynamics impacting outcome in non-small cell lung cancer patients treated with immune checkpoint inhibitor monotherapy.Front Immunol. 2024 Aug 21;15:1444007. doi: 10.3389/fimmu.2024.1444007. eCollection 2024. Front Immunol. 2024. PMID: 39238637 Free PMC article.
-
Multiplex single-cell profiling of putative cancer stem cell markers ALDH1, SOX9, SOX2, CD44, CD133 and CD15 in endometrial cancer.Mol Oncol. 2025 Jun;19(6):1651-1667. doi: 10.1002/1878-0261.13815. Epub 2025 Jan 31. Mol Oncol. 2025. PMID: 39888143 Free PMC article.
-
Single-cell high-dimensional imaging mass cytometry: one step beyond in oncology.Semin Immunopathol. 2023 Jan;45(1):17-28. doi: 10.1007/s00281-022-00978-w. Epub 2023 Jan 4. Semin Immunopathol. 2023. PMID: 36598557 Free PMC article. Review.
-
An Organoid Model for Translational Cancer Research Recapitulates Histoarchitecture and Molecular Hallmarks of Non-Small-Cell Lung Cancer.Cancers (Basel). 2025 Jun 3;17(11):1873. doi: 10.3390/cancers17111873. Cancers (Basel). 2025. PMID: 40507353 Free PMC article.
-
Architectural organization and molecular profiling of 3D cancer heterospheroids and their application in drug testing.Front Oncol. 2024 Jul 1;14:1386097. doi: 10.3389/fonc.2024.1386097. eCollection 2024. Front Oncol. 2024. PMID: 39011470 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

