Multimodality Characterization of Cancer-Associated Fibroblasts in Tumor Microenvironment and Its Correlation With Ultrasound Shear Wave-Measured Tissue Stiffness in Localized Prostate Cancer
- PMID: 35530322
- PMCID: PMC9069005
- DOI: 10.3389/fonc.2022.822476
Multimodality Characterization of Cancer-Associated Fibroblasts in Tumor Microenvironment and Its Correlation With Ultrasound Shear Wave-Measured Tissue Stiffness in Localized Prostate Cancer
Abstract
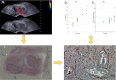
Introduction: Growing evidence suggests that the tumor microenvironment (TME) represented by cellular and acellular components plays a key role in the multistep process of metastases and response to therapies. However, imaging and molecular characterization of the TME in prostate cancer (PCa) and its role in predicting aggressive tumor behavior and disease progression is largely unexplored. The study explores the PCa TME through the characterization of cancer-associated fibroblasts (CAFs) using both immunohistochemistry (IHC) and genomics approaches. This is then correlated with transrectal ultrasound shear wave elastography (USWE)-measured tissue stiffness.
Patients and methods: Thirty patients with clinically localized PCa undergoing radical prostatectomy for different risk categories of tumor (low, intermediate, and high) defined by Gleason score (GS) were prospectively recruited into this study. Prostatic tissue stiffness was measured using USWE prior to surgery. The CAFs within the TME were identified by IHC using a panel of six antibodies (FAP, SMAα, FSP1, CD36, PDGFRα, and PDGFRβ) as well as gene expression profiling using TempO-sequence analysis. Whether the pattern and degree of immunohistochemical positivity (measured by Quick score method) and expression of genes characterizing CAFs were correlated with USWE- and GS-measured tissue stiffnesses were tested using Spearman's rank correlation and Pearson correlation.
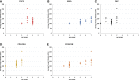
Results: There was a statistically significant correlation between GS of cancers, the pattern of staining for CAFs by immunohistochemical staining, and tissue stiffness measured in kPa using USWE (p < 0.001). Significant differences were also observed in immunohistochemical staining patterns between normal prostate and prostatic cancerous tissue. PDGFRβ and SMAα immunostaining scores increased linearly with increasing the USWE stiffness and the GS of PCa. There was a significant positive correlation between increasing tissue stiffness in tumor stroma and SMAα and PDGFRβ gene expression in the fibromuscular stroma (p < 0.001).
Conclusion: USWE-measured tissue stiffness correlates with increased SMAα and PDGFRβ expressing CAFs and PCa GSs. This mechanistic correlation could be used for predicting the upgrading of GS from biopsies to radical surgery and response to novel treatments.
Keywords: cancer-associated fibroblasts; immunohistochemistry; prostate cancer; stiffness; tumor microenvironment.
Copyright © 2022 Ageeli, Zhang, Ogbonnaya, Bray, Kernohan, Wilson, Li and Nabi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Quantitative ultrasound shear wave elastography (USWE)-measured tissue stiffness correlates with PIRADS scoring of MRI and Gleason score on whole-mount histopathology of prostate cancer: implications for ultrasound image-guided targeting approach.Insights Imaging. 2021 Jul 8;12(1):96. doi: 10.1186/s13244-021-01039-w. Insights Imaging. 2021. PMID: 34236553 Free PMC article.
-
Prostate Cancer Gleason Score From Biopsy to Radical Surgery: Can Ultrasound Shear Wave Elastography and Multiparametric Magnetic Resonance Imaging Narrow the Gap?Front Oncol. 2021 Nov 23;11:740724. doi: 10.3389/fonc.2021.740724. eCollection 2021. Front Oncol. 2021. PMID: 34888237 Free PMC article.
-
Utility of Gleason pattern 4 morphologies detected on transrectal ultrasound (TRUS)-guided biopsies for prediction of upgrading or upstaging in Gleason score 3 + 4 = 7 prostate cancer.Virchows Arch. 2016 Sep;469(3):313-9. doi: 10.1007/s00428-016-1981-2. Epub 2016 Jul 10. Virchows Arch. 2016. PMID: 27394432
-
Multiparametric MRI in detection and staging of prostate cancer.Dan Med J. 2017 Feb;64(2):B5327. Dan Med J. 2017. PMID: 28157066 Review.
-
Cancer-associated fibroblasts in radiotherapy: Bystanders or protagonists?Cell Commun Signal. 2023 May 11;21(1):108. doi: 10.1186/s12964-023-01093-5. Cell Commun Signal. 2023. PMID: 37170098 Free PMC article. Review.
Cited by
-
Ultrasound-based radiogenomics: status, applications, and future direction.Ultrasonography. 2025 Mar;44(2):95-111. doi: 10.14366/usg.24152. Epub 2024 Dec 12. Ultrasonography. 2025. PMID: 39935290 Free PMC article.
-
Fiber Bundle Image Reconstruction Using Convolutional Neural Networks and Bundle Rotation in Endomicroscopy.Sensors (Basel). 2023 Feb 23;23(5):2469. doi: 10.3390/s23052469. Sensors (Basel). 2023. PMID: 36904673 Free PMC article.
-
Advances in landscape and related therapeutic targets of the prostate tumor microenvironment.Acta Biochim Biophys Sin (Shanghai). 2023 Jun 9;55(6):956-973. doi: 10.3724/abbs.2023092. Acta Biochim Biophys Sin (Shanghai). 2023. PMID: 37294106 Free PMC article. Review.
References
-
- Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive Stroma in Human Prostate Cancer: Induction of Myofibroblast Phenotype and Extracellular Matrix Remodeling. Clin Cancer Res (2002) 8:2912–23. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

