Fabrication of gelatin Bi2S3 capsules as a highly sensitive X-ray contrast agent for gastrointestinal motility assessment in vivo
- PMID: 35530383
- PMCID: PMC9069310
- DOI: 10.1039/d2ra00993e
Fabrication of gelatin Bi2S3 capsules as a highly sensitive X-ray contrast agent for gastrointestinal motility assessment in vivo
Abstract
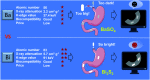
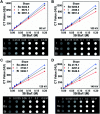
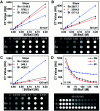
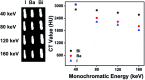
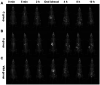
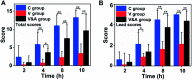
Tiny BaSO4 rod-based X-ray imaging is the most frequently-used method for clinical diagnosis of gastrointestinal motility disorders. The BaSO4 rods usually have a small size to pass through the gastrointestinal tract smoothly, but suffer from unavoidably low sensitivity. Herein, we developed Bi2S3 capsules as a high-performance X-ray contrast agent for gastrointestinal motility assessment for the first time. The Bi2S3 capsules were synthesized by the encapsulation of commercial Bi2S3 powder into commercial gelatin capsules and subsequent coating of ultraviolet-curable resin. The prepared Bi2S3 capsules showed excellent biocompatibility in vitro and in vivo and superior X-ray attenuation ability due to the large atomic number and high K-edge value of Bi. The developed Bi2S3 capsules can serve as a small but highly sensitive X-ray contrast agent to quantitatively assess gastrointestinal motility in a vincristine-induced gastrointestinal motility disorder model in vivo by X-ray, CT and spectral CT imaging successfully, solving the intrinsic drawbacks of clinically used BaSO4.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Bytzer P. Talley N. J. Leemon M. Arch. Intern. Med. 2001;161:1989–1996. - PubMed
LinkOut - more resources
Full Text Sources

