Osteopontin enhances sperm capacitation and in vitro fertilization efficiency in boars
- PMID: 35530410
- PMCID: PMC9039945
- DOI: 10.5187/jast.2022.e15
Osteopontin enhances sperm capacitation and in vitro fertilization efficiency in boars
Abstract
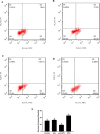
In this study, we used more reliable experimental materials and methods to detect the effects of osteopontin (OPN) on boar sperm in vitro capacitation, acrosome reaction, and fertilization efficiency. We reorganized and obtained the OPN protein of the porcine source. Immunofluorescence and Western blot show the localization and expression of the OPN protein before and after sperm capacitation. To determine whether OPN can affect sperm during sperm capacitation, we examined cyclic adenosine monophosphate (cAMP) concentrations after sperm capacitation, and the results showed that OPN significantly increased the cAMP concentration in sperm (p < 0.05). Flow cytometry showed that 0.1 μg/mL OPN-treated sperm had better acrosome reaction ability. In vitro fertilization (IVF) showed that 0.1 μg/mL OPN significantly increased the rate of embryo division. In conclusion, this study found that 0.1 μg/mL porcine OPN protein can significantly improve porcine capacitated sperm motility, cAMP concentration after capacitation sperm, acrosome reaction ability, and embryo division during IVF and provides new clues to explore the mechanism of OPN's function on sperm.
Keywords: Acrosome reaction; Capacitation; In vitro fertilization; Osteopontin; Sperm.
© Copyright 2022 Korean Society of Animal Science and Technology.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

Similar articles
-
Osteopontin improves sperm capacitation and in vitro fertilization efficiency in buffalo (Bubalus bubalis).Theriogenology. 2013 Aug;80(3):212-7. doi: 10.1016/j.theriogenology.2013.04.017. Epub 2013 May 29. Theriogenology. 2013. PMID: 23726297
-
Effect of osteopontin (OPN) on in vitro embryo development in cattle.Theriogenology. 2009 Feb;71(3):450-7. doi: 10.1016/j.theriogenology.2008.08.012. Epub 2008 Oct 4. Theriogenology. 2009. PMID: 18835636
-
GPx6 is involved in the in vitro induced capacitation and acrosome reaction in porcine sperm.Theriogenology. 2020 Oct 15;156:107-115. doi: 10.1016/j.theriogenology.2020.06.020. Epub 2020 Jun 29. Theriogenology. 2020. PMID: 32698036
-
[Why and how to ameliorate capacitation without inducing an acrosome reaction?].Contracept Fertil Sex. 1994 May;22(5):319-24. Contracept Fertil Sex. 1994. PMID: 8032388 Review. French.
-
The meaning of sperm capacitation. A historical perspective.J Androl. 1984 Mar-Apr;5(2):45-50. doi: 10.1002/j.1939-4640.1984.tb00775.x. J Androl. 1984. PMID: 6370941 Review.
References
-
- Elmi A, Banchelli F, Barone F, Fantinati P, Ventrella D, Forni M, et al. Semen evaluation and in vivo fertility in a Northern Italian pig farm: can advanced statistical approaches compensate for low sample size? An observational study. Anim Reprod Sci. 2018;192:61–8. doi: 10.1016/j.anireprosci.2018.02.014. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

