Synthesis and application of Bi2WO6 for the photocatalytic degradation of two typical fluoroquinolones under visible light irradiation
- PMID: 35530482
- PMCID: PMC9070762
- DOI: 10.1039/c9ra04445k
Synthesis and application of Bi2WO6 for the photocatalytic degradation of two typical fluoroquinolones under visible light irradiation
Abstract
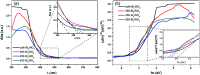
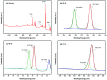
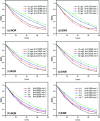
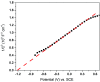
Bismuth tungstate (Bi2WO6) was successfully synthesized by a method combining ultrasonic solvothermal treatment and high-temperature calcination. The products were affirmed by X-ray diffraction, scanning electron microscopy, UV-vis diffuse reflection spectroscopy, X-ray photoelectron spectroscopy, and Fourier transform infrared spectroscopy. The characterization results indicated that calcination could improve the crystallinity and visible light utilization capacity of Bi2WO6. The photodegradation experiments showed that Bi2WO6 calcined at 450 °C for 3 h exhibited better photocatalytic activity for the degradation of norfloxacin and enrofloxacin under visible light irradiation than the catalyst prepared without calcination or calcined at other temperatures. Meanwhile, the effects of the amount of 450-Bi2WO6, the initial concentration of targets, and the pH of the solutions on the degradation were studied. Under the optimal conditions, the removal ratios reached to 92.95% (for norfloxacin) and 94.58% (for enrofloxacin) within 75 min. Furthermore, h+ and ·O2 - were identified to affect the photodegradation process significantly, and the possible photocatalytic mechanism was proposed. The as-prepared sample was verified to possess good stability and reusability, suggesting its potential application prospect in the treatment of fluoroquinolone antibiotics.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Wang J. J. Tang L. Zeng G. M. Zhou Y. Y. Deng Y. C. Fan C. Z. Gong J. L. Liu Y. N. Trans. Nonferrous Met. Soc. China. 2017;27:1794–1803.
LinkOut - more resources
Full Text Sources

