An erythrocyte membrane coated mimetic nano-platform for chemo-phototherapy and multimodal imaging
- PMID: 35530495
- PMCID: PMC9070788
- DOI: 10.1039/c9ra05867b
An erythrocyte membrane coated mimetic nano-platform for chemo-phototherapy and multimodal imaging
Abstract
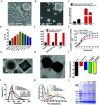
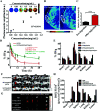
The tumor variability and low efficiency associated with conventional chemical drugs provide an impetus to develop drug-carrying systems with targeted accumulation and controllable release behavior. Herein, DOX-loaded Prussian blue (PB) nano-composites are developed after coating with erythrocyte membrane (EM) and modifying with folic acid (FA). In these nano-composites, PB nanoparticles mixture with different shapes were adopted to improve the photothermal performance, which is highly helpful for cancer photothermal ablation and controllable drug release. In addition, the nano-composites were endowed with high biocompatibility, immune evading capacity, pH-/photo-responsive release behavior, and markedly prolonged blood circulation time, which was reflected by a 99.6% cervical tumor growth inhibition value (TGI) in vivo. Meanwhile, they functioned as multimodal bioimaging agents for photothermal, fluorescence, and photoacoustic imaging of tumors. The reported strategy can be applied for personalized therapy of various tumors by modifying the tumor-targeting molecule on the surface of nanoparticles.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






Similar articles
-
Dual Chemodrug-Loaded Single-Walled Carbon Nanohorns for Multimodal Imaging-Guided Chemo-Photothermal Therapy of Tumors and Lung Metastases.Theranostics. 2018 Feb 15;8(7):1966-1984. doi: 10.7150/thno.23848. eCollection 2018. Theranostics. 2018. PMID: 29556368 Free PMC article.
-
Thermo- and pH-dual responsive polymeric micelles with upper critical solution temperature behavior for photoacoustic imaging-guided synergistic chemo-photothermal therapy against subcutaneous and metastatic breast tumors.Theranostics. 2018 Jul 16;8(15):4097-4115. doi: 10.7150/thno.26195. eCollection 2018. Theranostics. 2018. PMID: 30128039 Free PMC article.
-
Folic acid-modified Prussian blue/polydopamine nanoparticles as an MRI agent for use in targeted chemo/photothermal therapy.Biomater Sci. 2019 Jul 1;7(7):2996-3006. doi: 10.1039/c9bm00276f. Epub 2019 May 21. Biomater Sci. 2019. PMID: 31111139
-
Dual pH/reduction-responsive hybrid polymeric micelles for targeted chemo-photothermal combination therapy.Acta Biomater. 2018 Jul 15;75:371-385. doi: 10.1016/j.actbio.2018.05.026. Epub 2018 May 17. Acta Biomater. 2018. PMID: 29777957
-
Nanosized Prussian blue and its analogs for bioimaging and cancer theranostics.Acta Biomater. 2024 Mar 1;176:77-98. doi: 10.1016/j.actbio.2023.12.047. Epub 2024 Jan 2. Acta Biomater. 2024. PMID: 38176673 Review.
Cited by
-
Cell Membrane-Camouflaged Nanocarriers for Cancer Diagnostic and Therapeutic.Front Pharmacol. 2020 Feb 4;11:24. doi: 10.3389/fphar.2020.00024. eCollection 2020. Front Pharmacol. 2020. PMID: 32116701 Free PMC article. Review.
-
Phototheranostics Using Erythrocyte-Based Particles.Biomolecules. 2021 May 13;11(5):729. doi: 10.3390/biom11050729. Biomolecules. 2021. PMID: 34068081 Free PMC article. Review.
-
Research Progress of Photothermal Nanomaterials in Multimodal Tumor Therapy.Front Oncol. 2022 Jul 6;12:939365. doi: 10.3389/fonc.2022.939365. eCollection 2022. Front Oncol. 2022. PMID: 35898892 Free PMC article. Review.
-
Nano-Biomimetic Drug Delivery Vehicles: Potential Approaches for COVID-19 Treatment.Molecules. 2020 Dec 16;25(24):5952. doi: 10.3390/molecules25245952. Molecules. 2020. PMID: 33339110 Free PMC article. Review.
-
Cell membrane camouflaged biomimetic nanoparticles: Focusing on tumor theranostics.Mater Today Bio. 2022 Mar 1;14:100228. doi: 10.1016/j.mtbio.2022.100228. eCollection 2022 Mar. Mater Today Bio. 2022. PMID: 35265826 Free PMC article.
References
-
- Chen W. Zeng K. Liu H. Ouyang J. Wang L. Liu Y. et al., Cell Membrane Camouflaged Hollow Prussian Blue Nanoparticles for Synergistic Photothermal-/Chemotherapy of Cancer. Adv. Funct. Mater. 2017;27:1605795. doi: 10.1002/adfm.201605795. - DOI
LinkOut - more resources
Full Text Sources

