Advances in Our Clinical Understanding of Autonomic Regulation Therapy Using Vagal Nerve Stimulation in Patients Living With Heart Failure
- PMID: 35530511
- PMCID: PMC9068946
- DOI: 10.3389/fphys.2022.857538
Advances in Our Clinical Understanding of Autonomic Regulation Therapy Using Vagal Nerve Stimulation in Patients Living With Heart Failure
Abstract
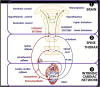
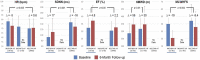
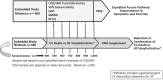
The ANTHEM-HF, INOVATE-HF, and NECTAR-HF clinical studies of autonomic regulation therapy (ART) using vagus nerve stimulation (VNS) systems have collectively provided dose-ranging information enabling the development of several working hypotheses on how stimulation frequency can be utilized during VNS for tolerability and improving cardiovascular outcomes in patients living with heart failure (HF) and reduced ejection fraction (HFrEF). Changes in heart rate dynamics, comprising reduced heart rate (HR) and increased HR variability, are a biomarker of autonomic nerve system engagement and cardiac control, and appear to be sensitive to VNS that is delivered using a stimulation frequency that is similar to the natural operating frequency of the vagus nerve. Among prior studies, the ANTHEM-HF Pilot Study has provided the clearest evidence of autonomic engagement with VNS that was delivered using a stimulation frequency that was within the operating range of the vagus nerve. Achieving autonomic engagement was accompanied by improvement from baseline in six-minute walk duration (6MWD), health-related quality of life, and left ventricular EF (LVEF), over and above those achieved by concomitant guideline-directed medical therapy (GDMT) administered to counteract harmful neurohormonal activation, with relative freedom from deleterious effects. Autonomic engagement and positive directional changes have persisted over time, and an exploratory analysis suggests that improvement in autonomic tone, symptoms, and physical capacity may be independent of baseline NT-proBNP values. Based upon these encouraging observations, prospective, randomized controlled trials examining the effects on symptoms and cardiac function as well as natural history have been warranted. A multi-national, large-scale, randomized, controlled trial is well underway to determine the outcomes associated with ART using autonomic nervous system engagement as a guide for VNS delivery.
Keywords: autonomic nervous system; autonomic regulation therapy; cardiomyopathy; guideline directed medical therapy (GDMT); heart failure; left ventricular ejection fraction; neuromodulation; vagus nerve stimulation.
Copyright © 2022 Konstam, Mann, Udelson, Ardell, De Ferrari, Cowie, Klein, Gregory, Massaro, Libbus, DiCarlo, Butler, Parker and Teerlink.
Conflict of interest statement
DG is a biostatistician and has been an employee of Cardiovascular Clinical Studies Foundation LLC (CCSF), the contract research organization (CRO) that has been contracted by LivaNova, United States Incorporated, for ANTHEM-HFrEF Pivotal Study operations. JU is contracted to CCSF as a cardiovascular consultant. JM is contracted to CCSF for statistical consultation. MK is contracted to CCSF and LivaNova, respectively, as a cardiovascular consultant. JA is contracted to LivaNova as a neurocardiology consultant. JB, HK, MK, DM, GD, JP, JT, and JU are contracted to LivaNova as members of the ANTHEM-HFrEF Pivotal Study Steering Committee. IL and LD are employees and shareholders of LivaNova, United States Incorporated. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Comparison of symptomatic and functional responses to vagus nerve stimulation in ANTHEM-HF, INOVATE-HF, and NECTAR-HF.ESC Heart Fail. 2020 Feb;7(1):75-83. doi: 10.1002/ehf2.12592. Epub 2020 Jan 27. ESC Heart Fail. 2020. PMID: 31984682 Free PMC article.
-
Persistent Autonomic Engagement and Cardiac Control After Four or More Years of Autonomic Regulation Therapy Using Vagus Nerve Stimulation.Front Physiol. 2022 Mar 11;13:853617. doi: 10.3389/fphys.2022.853617. eCollection 2022. Front Physiol. 2022. PMID: 35360224 Free PMC article.
-
Baseline NT-proBNP and responsiveness to autonomic regulation therapy in patients with heart failure and reduced ejection fraction.Int J Cardiol Heart Vasc. 2020 May 30;29:100520. doi: 10.1016/j.ijcha.2020.100520. eCollection 2020 Aug. Int J Cardiol Heart Vasc. 2020. PMID: 32509959 Free PMC article.
-
Non-pharmacologic autonomic neuromodulation for treatment of heart failure: A systematic review and meta-analysis of randomized controlled trials.Trends Cardiovasc Med. 2024 Feb;34(2):101-107. doi: 10.1016/j.tcm.2022.09.007. Epub 2022 Oct 4. Trends Cardiovasc Med. 2024. PMID: 36202286
-
Vagal stimulation in heart failure.Herz. 2021 Dec;46(6):541-549. doi: 10.1007/s00059-021-05076-5. Epub 2021 Oct 30. Herz. 2021. PMID: 34716778 Free PMC article. Review.
Cited by
-
Transcriptional response of the heart to vagus nerve stimulation.Physiol Genomics. 2024 Feb 1;56(2):167-178. doi: 10.6084/m9.figshare.24449590. Epub 2023 Dec 4. Physiol Genomics. 2024. PMID: 39071113 Free PMC article.
-
Sympathetic Nervous System in Heart Failure: Targets for Treatments.Curr Hypertens Rep. 2025 Jul 21;27(1):20. doi: 10.1007/s11906-025-01337-4. Curr Hypertens Rep. 2025. PMID: 40691427 Review.
-
Vagus Nerve Stimulation and Its Cardioprotective Abilities: A Systematic Review.J Clin Med. 2023 Feb 21;12(5):1717. doi: 10.3390/jcm12051717. J Clin Med. 2023. PMID: 36902505 Free PMC article. Review.
-
Sympathetic components in left and right human cervical vagus nerve: implications for vagus nerve stimulation.Front Neuroanat. 2023 Jul 10;17:1205660. doi: 10.3389/fnana.2023.1205660. eCollection 2023. Front Neuroanat. 2023. PMID: 37492698 Free PMC article.
-
Transcriptional response of the heart to vagus nerve stimulation.Physiol Genomics. 2024 Feb 1;56(2):167-178. doi: 10.1152/physiolgenomics.00095.2023. Epub 2023 Dec 4. Physiol Genomics. 2024. PMID: 38047311 Free PMC article.
References
-
- Anand I., Libbus I., DiCarlo L. (2020). Long-Term Lead Performance for Vagus Nerve Stimulation: Low Rate of Complications and Failures. Nr 7, 26–29. 10.15540/nr.7.1.26 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

