Structural elaboration of dicyanopyrazine: towards push-pull molecules with tailored photoredox activity
- PMID: 35530614
- PMCID: PMC9069489
- DOI: 10.1039/c9ra04731j
Structural elaboration of dicyanopyrazine: towards push-pull molecules with tailored photoredox activity
Abstract
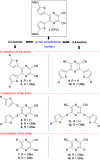
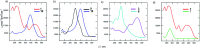
As an extension of the successful dicyanopyrazine photoredox catalysts, a series of X-shaped push-pull molecules with a systematically altered structure were designed and facilely synthesized; their structure-property relationship was elucidated in detail via experimental as well as theoretical calculations. Dicyanopyrazines are proven to be powerful photoredox catalysts with a push-pull arrangement that allows facile property tuning by interchanging a particular part of the D-π-A system. Changing the mutual position of the cyano acceptors and the methoxy, methylthio and thienyl donors as well as modifying the linker allowed wide tuning of the fundamental properties of the catalysts. Contrary to the currently available organic photoredox catalysts, we provided a series of catalysts based on a pyrazine heterocyclic scaffold with easy synthesis and further modification, diverse photoredox characteristics and wide application potential across modern photoredox transformations. The photoredox catalytic activities of the target catalysts were examined in a benchmark cross-dehydrogenative coupling and novel and challenging annulation reactions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Dicyanobenzene and dicyanopyrazine derived X-shaped charge-transfer chromophores: comparative and structure-property relationship study.Org Biomol Chem. 2014 Aug 7;12(29):5517-27. doi: 10.1039/c4ob00901k. Org Biomol Chem. 2014. PMID: 24948026
-
A Toolbox Approach To Construct Broadly Applicable Metal-Free Catalysts for Photoredox Chemistry: Deliberate Tuning of Redox Potentials and Importance of Halogens in Donor-Acceptor Cyanoarenes.J Am Chem Soc. 2018 Nov 14;140(45):15353-15365. doi: 10.1021/jacs.8b08933. Epub 2018 Nov 2. J Am Chem Soc. 2018. PMID: 30277767
-
Small isomeric push-pull chromophores based on thienothiophenes with tunable optical (non)linearities.Org Biomol Chem. 2019 Apr 3;17(14):3623-3634. doi: 10.1039/c9ob00487d. Org Biomol Chem. 2019. PMID: 30916108
-
Lead-halides Perovskite Visible Light Photoredox Catalysts for Organic Synthesis.Chem Rec. 2020 Oct;20(10):1181-1197. doi: 10.1002/tcr.202000049. Epub 2020 Aug 31. Chem Rec. 2020. PMID: 32865302 Review.
-
Photoredox Chemistry with Organic Catalysts: Role of Computational Methods.ACS Omega. 2021 Dec 3;6(49):33253-33264. doi: 10.1021/acsomega.1c05787. eCollection 2021 Dec 14. ACS Omega. 2021. PMID: 34926877 Free PMC article. Review.
Cited by
-
Selective functionalization of the 1H-imidazo[1,2-b]pyrazole scaffold. A new potential non-classical isostere of indole and a precursor of push-pull dyes.Chem Sci. 2021 Aug 30;12(39):12993-13000. doi: 10.1039/d1sc04155j. eCollection 2021 Oct 13. Chem Sci. 2021. PMID: 34745530 Free PMC article.
-
Aerobic Oxidative EDA Catalysis: Synthesis of Tetrahydroquinolines Using an Organocatalytic EDA Active Acceptor.J Org Chem. 2022 Jan 21;87(2):1457-1469. doi: 10.1021/acs.joc.1c02776. Epub 2022 Jan 10. J Org Chem. 2022. PMID: 35005960 Free PMC article.
References
-
- Lechner B. König B. Synthesis. 2010:1712.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

