Iodine-mediated synthesis of benzo[ a]fluorenones from yne-enones
- PMID: 35530623
- PMCID: PMC9069471
- DOI: 10.1039/c9ra02376c
Iodine-mediated synthesis of benzo[ a]fluorenones from yne-enones
Abstract
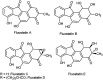
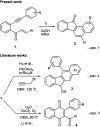
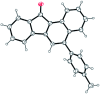
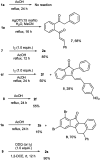
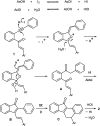
The chalcones derived from o-alkynylacetophenones and aromatic aldehydes (yne-enones) when heated under reflux with iodine in acetic acid gave a range of benzo[a]fluorenone derivatives in moderate to good yields. The transformation involves the formation of a vinyl indenone intermediate through regioselective alkyne hydration and intramolecular aldol condensation followed by electrocyclic ring closure and aromatization.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Divergent synthesis of 3,4-dihydro-2H-benzo[h]chromen-2-one and fluorenone derivatives from ortho-alkynylarylketones.Org Biomol Chem. 2023 Nov 15;21(44):8888-8901. doi: 10.1039/d3ob01492d. Org Biomol Chem. 2023. PMID: 37902976
-
Tandem synthesis of pyrroloacridones via [3 + 2] alkyne annulation/ring-opening with concomitant intramolecular aldol condensation.J Org Chem. 2013 Jun 7;78(11):5372-84. doi: 10.1021/jo400539x. Epub 2013 May 14. J Org Chem. 2013. PMID: 23642218
-
Yne-Enones Enable Diversity-Oriented Catalytic Cascade Reactions: A Rapid Assembly of Complexity.Acc Chem Res. 2020 Oct 20;53(10):2358-2371. doi: 10.1021/acs.accounts.0c00466. Epub 2020 Sep 30. Acc Chem Res. 2020. PMID: 32998506
-
Brønsted Acid-promoted intramolecular carbocyclization of alkynals leading to cyclic enones.Org Lett. 2009 Apr 2;11(7):1531-3. doi: 10.1021/ol900142r. Org Lett. 2009. PMID: 19245263
-
Brønsted/Lewis Acid-Promoted Site-Selective Intramolecular Cycloisomerizations of Aryl-Fused 1,6-Diyn-3-ones for Diversity-Oriented Synthesis of Benzo-Fused Fluorenes and Fluorenones and Naphthyl Ketones.J Org Chem. 2021 Jan 1;86(1):333-351. doi: 10.1021/acs.joc.0c02131. Epub 2020 Nov 30. J Org Chem. 2021. PMID: 33253563
Cited by
-
Iodine-catalyzed efficient synthesis of xanthene/thioxanthene-indole derivatives under mild conditions.RSC Adv. 2020 Jul 2;10(42):25165-25169. doi: 10.1039/d0ra05217e. eCollection 2020 Jun 29. RSC Adv. 2020. PMID: 35517437 Free PMC article.
References
-
- Baur S. Niehaus J. Karagouni A. D. Katsifas E. A. Chalkou K. Meintanis C. Jones A. L. Goodfellow M. Ward A. C. Beil W. Schneider K. Sussmuth R. D. Fiedler H. P. J. Antibiot. 2006;59:293–297. doi: 10.1038/ja.2006.41. - DOI - PubMed
- Feng Z. Y. Kim J. H. Brady S. F. J. Am. Chem. Soc. 2010;132:11902–11903. doi: 10.1021/ja104550p. - DOI - PMC - PubMed
- Zhang G. Y. Zhang W. M. Liu J. S. Zhang C. S. J. Nat. Prod. 2012;75:1937–1943. doi: 10.1021/np300505y. - DOI - PubMed
-
- Crowell T. A., Jones C. A. and Bryant H. U., US 5958917 A, September 28, 1999
- Zheng S., Vaeth K. M. and Bennett G. A., US 6849348 B2, February 1, 2005
-
- Laufer R. S. Dmitrienko G. I. J. Am. Chem. Soc. 2002;124:1854–1855. doi: 10.1021/ja0167809. - DOI - PubMed
- Liu W. Buck M. Chen N. Shang M. Taylor N. J. Asoud J. Wu X. Hasinoff B. B. Dmitrienko G. I. Org. Lett. 2007;9:2915–2918. doi: 10.1021/ol0712374. - DOI - PubMed
- Zhao Y.-B. Mariampillai B. Candito D. A. Laleu B. Li M. Lautens M. Angew. Chem., Int. Ed. 2009;48:1849–1852. doi: 10.1002/anie.200805780. - DOI - PubMed
LinkOut - more resources
Full Text Sources

