High-throughput metabolomics for evaluating the efficacy and discovering the metabolic mechanism of Luozhen capsules from the excessive liver-fire syndrome of hypertension
- PMID: 35530762
- PMCID: PMC9072971
- DOI: 10.1039/c9ra06622e
High-throughput metabolomics for evaluating the efficacy and discovering the metabolic mechanism of Luozhen capsules from the excessive liver-fire syndrome of hypertension
Abstract
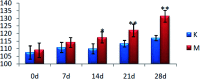
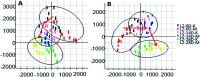
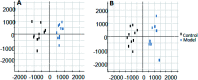
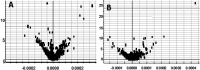
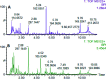
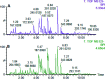
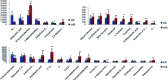
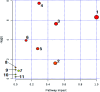
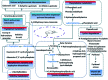
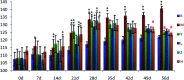
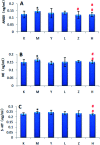
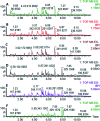
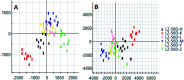
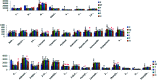
Essential hypertension (EH) is a chronic disease characterized by a variety of causes of elevated systemic arterial pressure, which often causes functional or organic damage to important organs such as the heart, brain, and kidney. Hypertension of excessive liver-fire syndrome is a type of classification for young people with essential hypertension. The disease is slower in its onset and its symptoms are more ambiguous, and thus its pathogenesis is complicated and still unclear. In this study, aconite, dried ginger and cinnamon extracts were combined with l-NAME to establish a model of excessive liver-fire hypertension. Blood pressure (systolic blood pressure), ANGII, NE and 5-HT were used as evaluation indicators to establish the model. Urinary metabolomics based on ultra-high performance liquid chromatography coupled with quadruple time-of-flight mass spectrometry was used to characterize the metabolic changes and potential biomarkers in modeled rats. Compared to the treatment group, 32 potential biomarkers were initially identified in the model using multivariate statistical analysis involving 11 metabolic pathways. After oral administration of Luozhen capsules, eight biomarkers that can be adjusted in high, medium and low doses of Luozhen capsules in urine were preliminarily determined, mainly involving two metabolic pathways of amino acid metabolism and lipid metabolism. In conclusion, this study explored the metabolomic changes in rats with hypertension of liver-fire hyperactivity syndrome and the post-dose metabolomics, determined the relevant biomarker groups, and clarified the metabonomic connotation of Luozhen capsules in the treatment of liver-fire excessive type hypertension.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
[Metabolomics study of urine with Benzene, Toluene and Xylene combined exposure based on ultra performance liquid chromatography-quadrupole time-of-flight mass spectrometry].Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2021 Apr 20;39(4):248-252. doi: 10.3760/cma.j.cn121094-20200228-00092. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2021. PMID: 33910281 Chinese.
-
Identification of essential hypertension biomarkers in human urine by non-targeted metabolomics based on UPLC-Q-TOF/MS.Clin Chim Acta. 2018 Nov;486:192-198. doi: 10.1016/j.cca.2018.08.006. Epub 2018 Aug 7. Clin Chim Acta. 2018. PMID: 30092170 Clinical Trial.
-
Urinary metabonomic study of Panax ginseng in deficiency of vital energy rat using ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry.J Ethnopharmacol. 2016 May 26;184:10-7. doi: 10.1016/j.jep.2016.02.031. Epub 2016 Feb 24. J Ethnopharmacol. 2016. PMID: 26921673
-
Metabolomics analysis of Semen Cuscutae protection of kidney deficient model rats using ultra high-performance liquid chromatography-quadrupole time-of-flight Mass Spectrometry.J Pharm Biomed Anal. 2022 Jan 5;207:114432. doi: 10.1016/j.jpba.2021.114432. Epub 2021 Oct 20. J Pharm Biomed Anal. 2022. PMID: 34715580
-
Identification of biomarkers for essential hypertension based on metabolomics.Nutr Metab Cardiovasc Dis. 2021 Feb 8;31(2):382-395. doi: 10.1016/j.numecd.2020.11.023. Epub 2020 Dec 2. Nutr Metab Cardiovasc Dis. 2021. PMID: 33495028
Cited by
-
Metabolomics biotechnology, applications, and future trends: a systematic review.RSC Adv. 2019 Nov 14;9(64):37245-37257. doi: 10.1039/c9ra06697g. eCollection 2019 Nov 13. RSC Adv. 2019. PMID: 35542267 Free PMC article. Review.
-
Characteristics and needs of physical and mental health among older adult individuals with disabilities under the background of smart healthcare: according to data from the China family panel studies.Front Public Health. 2025 Feb 21;13:1475466. doi: 10.3389/fpubh.2025.1475466. eCollection 2025. Front Public Health. 2025. PMID: 40061462 Free PMC article.
-
Antihypertensive Activity of Milk Fermented by Lactiplantibacillus plantarum SR37-3 and SR61-2 in L-NAME-Induced Hypertensive Rats.Foods. 2022 Aug 4;11(15):2332. doi: 10.3390/foods11152332. Foods. 2022. PMID: 35954098 Free PMC article.
-
Comparative metabolomics unveils molecular changes and metabolic networks of syringin against hepatitis B mice by untargeted mass spectrometry.RSC Adv. 2020 Jan 2;10(1):461-473. doi: 10.1039/c9ra06332c. eCollection 2019 Dec 20. RSC Adv. 2020. PMID: 35492557 Free PMC article.
-
Exploring the metabolic biomarkers and pathway changes in crucian under carbonate alkalinity exposure using high-throughput metabolomics analysis based on UPLC-ESI-QTOF-MS.RSC Adv. 2020 Jan 9;10(3):1552-1571. doi: 10.1039/c9ra08090b. eCollection 2020 Jan 7. RSC Adv. 2020. PMID: 35494719 Free PMC article.
References
LinkOut - more resources
Full Text Sources

