The role of surface copper content on biofilm formation by drinking water bacteria
- PMID: 35530774
- PMCID: PMC9072912
- DOI: 10.1039/c9ra05880j
The role of surface copper content on biofilm formation by drinking water bacteria
Abstract
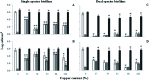
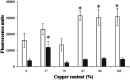
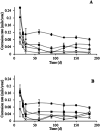
Copper pipes are conventionally used to supply tap water. Their role in biofilm prevention remains to be understood. This study evaluates the ability of selected surface materials with different copper contents (0, 57, 79, 87, 96, 100% of copper) to control biofilm formation and regrowth. Further experiments were performed to assess copper leaching and corrosion under conditions mimicking real plumbing systems. Acinetobacter calcoaceticus and Stenotrophomonas maltophilia isolated from a drinking water distribution system were used as model bacteria. All the copper materials showed positive results on the control of single and dual species biofilms presenting high reductions of bacterial culturability > 4 log CFU per cm2. The antimicrobial action of the selected materials seem not to be related to copper leaching or to the formation of reactive oxygen species. However, bacterial-copper contact demonstrated damage to bacterial membranes. The alloy containing 96% copper was the most promising surface in reducing biofilm culturability and viability, and was the only surface able to avoid the regrowth of single species biofilms when in contact with high nutrient concentrations. The alloy with 87% copper was shown to be unsuitable for use in chlorinated systems due to the high copper leaching observed when exposed to free chlorine. The presence of viable but non-culturable bacteria was remarkable, particularly in dual species biofilms. The overall results provide novel data on the role of copper alloys for use under chlorinated and unchlorinated conditions. Copper alloys demonstrated comparable or even higher biofilm control effects than elemental copper surfaces.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
Authors declare no conflict of interest.
Figures


References
-
- NRC, Drinking water distribution systems: Assessing and reducing risks, N.R.C., Washington, DC, 2006
-
- Critchley M. M. Cromar N. J. McClure N. Fallowfield H. J. Water Sci. Technol.: Water Supply. 2002;2:81–87.
-
- Simões L. C. Simões M. RSC Adv. 2013;3:2520–2533. doi: 10.1039/C2RA22243D. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

