Palladium-catalyzed oxidative cross-coupling for the synthesis of α-amino ketones
- PMID: 35530775
- PMCID: PMC9072988
- DOI: 10.1039/c9ra06108h
Palladium-catalyzed oxidative cross-coupling for the synthesis of α-amino ketones
Abstract
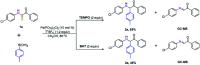
A novel oxidative cross-coupling reaction for the synthesis of α-aryl α-amino ketones in the presence of palladium catalysts using T+BF4 - as an oxidant has been developed. This transformation was achieved by direct C-H oxidation of α-aminocarbonyl compounds and arylation. The mild reaction has a broad reaction scope and gives desired α-aryl α-amino ketones in moderate to excellent yields.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Cho S. H. Kim J. Y. Kwak J. Chang S. Chem. Soc. Rev. 2011;40:5068–5083. doi: 10.1039/C1CS15082K. - DOI - PubMed
- Girard S. A. Knauber T. Li C.-J. Angew. Chem., Int. Ed. 2014;53:74–100. doi: 10.1002/anie.201304268. - DOI - PubMed
- Li C.-J. Acc. Chem. Res. 2009;42:335–344. doi: 10.1021/ar800164n. - DOI - PubMed
- Li C.-J. Li Z. Pure Appl. Chem. 2006;78:935.
- Scheuermann C. J. Chem.–Asian J. 2010;5:436–451. doi: 10.1002/asia.200900487. - DOI - PubMed
- Yeung C. S. Dong V. M. Chem. Rev. 2011;111:1215–1292. doi: 10.1021/cr100280d. - DOI - PubMed
- Cherney A. H. Kadunce N. T. Reisman S. E. Chem. Rev. 2015;115:9587–9652. doi: 10.1021/acs.chemrev.5b00162. - DOI - PMC - PubMed
- Newton C. G. Wang S.-G. Oliveira C. C. Cramer N. Chem. Rev. 2017;117:8908–8976. doi: 10.1021/acs.chemrev.6b00692. - DOI - PubMed
-
- Choudhary G. Peddinti R. K. Green Chem. 2011;13:276. doi: 10.1039/C0GC00830C. - DOI
- Gawande M. B. Bonifácio V. D. Luque R. Branco P. S. Varma R. S. Chem. Soc. Rev. 2013;42:5522. doi: 10.1039/C3CS60025D. - DOI - PubMed
- Jain S. L. Singhal S. Sain B. Green Chem. 2007;9:740. doi: 10.1039/B702311A. - DOI
- Khatik G. L. Kumar R. Chakraborti A. K. Org. Lett. 2006;8:2433. doi: 10.1021/ol060846t. - DOI - PubMed
- Reddy M. V. Kumar B. S. Lim K. T. Cho B. G. Jeong Y. T. Tetrahedron Lett. 2016;57:476. doi: 10.1016/j.tetlet.2015.12.061. - DOI
- Varma R. S. Green Chem. 1999;1:43. doi: 10.1039/A808223E. - DOI
- Zhang G. Zhang Y. Wang R. Angew. Chem., Int. Ed. 2011;50:10429–10432. doi: 10.1002/anie.201105123. - DOI - PubMed
- Zhang J. Cui Z. Wang F. Wang Y. Miao Z. Chen R. Green Chem. 2007;9:1341. doi: 10.1039/B710008F. - DOI
- Zhao L. Li C.-J. Angew. Chem., Int. Ed. 2008;47:7075–7078. doi: 10.1002/anie.200801367. - DOI - PubMed
- Zhu Z.-Q. Bai P. Huang Z.-Z. Org. Lett. 2014;16:4881–4883. doi: 10.1021/ol502402s. - DOI - PubMed
-
- Bodtke A. Langer P. Tetrahedron Lett. 2004;45:8741. doi: 10.1016/j.tetlet.2004.09.125. - DOI
- Bryan L. Richard A. Buzon K. F. Chiu T. Colgan M. L. Kasthurikrishnan N. Tetrahedron Lett. 2004;45:6887. doi: 10.1016/j.tetlet.2004.03.035. - DOI
- Chandrashaker V. Taniguchi M. Ptaszek M. Lindsey J. S. Tetrahedron. 2012;68:6957. doi: 10.1016/j.tet.2012.05.080. - DOI
- Medaer B. P. Hoornaert G. J. Tetrahedron. 1999;55:3987. doi: 10.1016/S0040-4020(99)00087-3. - DOI
- Murkute A. D. Jackson J. E. Miller D. J. J. Catal. 2011;278:189–199. doi: 10.1016/j.jcat.2010.12.001. - DOI
- Gellman S. H. Acc. Chem. Res. 1998;31:173–180. doi: 10.1021/ar960298r. - DOI
- Jose M. C. Humberto R.-S. Curr. Org. Chem. 2008;12:524–543. doi: 10.2174/138527208784245996. - DOI
- Klingler F. D. Acc. Chem. Res. 2007;40:1367–1376. doi: 10.1021/ar700100e. - DOI - PubMed
- Ohfune Y. Acc. Chem. Res. 1992;25:360–366. doi: 10.1021/ar00020a006. - DOI
- Farahi M. Tamaddon F. Karami B. Pasdar S. Tetrahedron Lett. 2015;56:1887–1890. doi: 10.1016/j.tetlet.2015.02.105. - DOI
- Tamaddon F. Dehghani Tafti A. Synlett. 2016;27:2217–2220. doi: 10.1055/s-0035-1561663. - DOI
-
- Xie J. Huang Z.-Z. Angew. Chem., Int. Ed. 2010;49:10181–10185. doi: 10.1002/anie.201004940. - DOI - PubMed
- Bandar J. S. Lambert T. H. J. Am. Chem. Soc. 2013;135:11799–11802. doi: 10.1021/ja407277a. - DOI - PMC - PubMed
- Binder J. T. Cordier C. J. Fu G. C. J. Am. Chem. Soc. 2012;134:17003–17006. doi: 10.1021/ja308460z. - DOI - PMC - PubMed
- Huo C. Wang C. Wu M. Jia X. Xie H. Yuan Y. Adv. Synth. Catal. 2014;356:411–415. doi: 10.1002/adsc.201300535. - DOI
- Huo C. Wu M. Chen F. Jia X. Yuan Y. Xie H. Chem. Commun. 2015;51:4708–4711. doi: 10.1039/C4CC09922B. - DOI - PubMed
- Huo C. Xie H. Chen F. Tang J. Wang Y. Adv. Synth. Catal. 2016;358:724–730. doi: 10.1002/adsc.201500893. - DOI
- Kotha S. Acc. Chem. Res. 2003;36:342–351. doi: 10.1021/ar020147q. - DOI - PubMed
- Nájera C. Sansano J. M. Chem. Rev. 2007;107:4584–4671. doi: 10.1021/cr050580o. - DOI - PubMed
- Saito S. Tsubogo T. Kobayashi S. J. Am. Chem. Soc. 2007;129:5364–5365. doi: 10.1021/ja0709730. - DOI - PubMed
- Salman M. Zhu Z.-Q. Huang Z.-Z. Org. Lett. 2016;18:1526–1529. doi: 10.1021/acs.orglett.6b00162. - DOI - PubMed
- Trost B. M. Miege F. J. Am. Chem. Soc. 2014;136:3016–3019. doi: 10.1021/ja4129394. - DOI - PMC - PubMed
- Wan M. Lou H. Liu L. Chem. Commun. 2015;51:13953–13956. doi: 10.1039/C5CC04791A. - DOI - PubMed
- Werner E. W. Mei T.-S. Burckle A. J. Sigman M. S. Science. 2012;338:1455–1458. doi: 10.1126/science.1229208. - DOI - PMC - PubMed
- Xue Z.-Y. Li Q.-H. Tao H.-Y. Wang C.-J. J. Am. Chem. Soc. 2011;133:11757–11765. doi: 10.1021/ja2043563. - DOI - PubMed
- Hui Y. Yilan X. Rui D. Yan F. Adv. Synth. Catal. 2017;359:39–43. doi: 10.1002/adsc.201600712. - DOI
- Wei X.-H. Wang G.-W. Yang S.-D. Chem. Commun. 2015;51:832–835. doi: 10.1039/C4CC07361D. - DOI - PubMed
- Wei X.-H. Zhao L.-B. Zhou H.-C. RSC Adv. 2017;7:16561–16564. doi: 10.1039/C7RA00664K. - DOI
- Wei Y. Wang J. Wang Y. Yao X. Yang C. Huo C. Org. Biomol. Chem. 2018;16:4985–4989. doi: 10.1039/C8OB01068D. - DOI - PubMed
- Zhang Y. Yang X. Zhou H. Li S. Zhu Y. Li Y. Org. Chem. Front. 2018;5:2120–2125. doi: 10.1039/C8QO00341F. - DOI
- Daggupati R. V. Malapaka C. Org. Chem. Front. 2018;5:788–792. doi: 10.1039/C7QO00851A. - DOI
- Revathi L. Ravindar L. Fang W.-Y. Rakesh K. P. Qin H.-L. Adv. Synth. Catal. 2018;360:4652–4698. doi: 10.1002/adsc.201800736. - DOI
- Wei X.-H. Zhao L.-B. Zhou H.-C. RSC Adv. 2017;7:16561–16564. doi: 10.1039/C7RA00664K. - DOI
- Zha G.-F. Bare G. A. L. Leng J. Shang Z.-P. Luo Z. Qin H.-L. Adv. Synth. Catal. 2017;359:3237–3242. doi: 10.1002/adsc.201700688. - DOI
- Zhang X. Rakesh K. P. Ravindar L. Qin H.-L. Green Chem. 2018;20:4790–4833. doi: 10.1039/C8GC02382D. - DOI
- Zhao C. Zha G.-F. Fang W.-Y. Rakesh K. P. Qin H.-L. Eur. J. Org. Chem. 2019;2019:1801–1807. doi: 10.1002/ejoc.201801888. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous