Therapeutic Effect of Teneligliptin in Drug-Induced Nephrotoxicity: An In-Vitro Study
- PMID: 35530894
- PMCID: PMC9074376
- DOI: 10.7759/cureus.23871
Therapeutic Effect of Teneligliptin in Drug-Induced Nephrotoxicity: An In-Vitro Study
Abstract
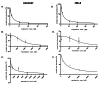
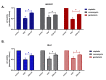
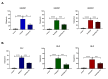
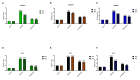
Background Drug-induced nephrotoxicity is an important side effect of many commonly used drugs. In this study, we planned to evaluate the effects of teneligliptin (TG), which is a dipeptidyl peptidase-4 (DPP-4) inhibitor, on cell healing by creating nephrotoxicity models in human renal proximal tubule cell and human embryonic kidney epithelial cells cell lines in-vitro with cisplatin, vancomycin, and gentamicin. Methodology First, we determined the 50% inhibitory concentration doses of nephrotoxic drugs and the nephroprotective dose of TG. Then, we analyzed the difference in cell viability, apoptosis, and oxidative stress (reactive oxygen and nitrogen species (ROS/RNS) production) between TG-treated and untreated cells after nephrotoxicity occurred. Moreover, we evaluated the expression of kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) in cells. Results We found that when cell lines were treated after toxicity was induced with TG, cell viability increased, apoptosis and ROS/RNS production were significantly decreased, and expressions of KIM-1 and NGAL were significantly reduced. Conclusions This study showed that TG has positive effects on the recovery of drug-induced nephrotoxicity in an in-vitro setting.
Keywords: cisplatin; drug-induced nephrotoxicity; gentamicin; teneligliptin; vancomycin.
Copyright © 2022, Becerir et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
The therapeutic effect of Cilastatin on drug-induced nephrotoxicity: a new perspective.Eur Rev Med Pharmacol Sci. 2021 Sep;25(17):5436-5447. doi: 10.26355/eurrev_202109_26651. Eur Rev Med Pharmacol Sci. 2021. PMID: 34533819
-
Dipeptidyl peptidase-4 inhibitor teneligliptin accelerates recovery from cisplatin-induced acute kidney injury by attenuating inflammation and promoting tubular regeneration.Nephrol Dial Transplant. 2019 Oct 1;34(10):1669-1680. doi: 10.1093/ndt/gfy397. Nephrol Dial Transplant. 2019. PMID: 30624740
-
In vitro evaluation of biomarkers for cisplatin-induced nephrotoxicity using HK-2 human kidney epithelial cells.Toxicol Lett. 2013 Mar 13;217(3):235-42. doi: 10.1016/j.toxlet.2012.12.015. Epub 2012 Dec 31. Toxicol Lett. 2013. PMID: 23287709
-
Unveiling drug induced nephrotoxicity using novel biomarkers and cutting-edge preventive strategies.Chem Biol Interact. 2024 Jan 25;388:110838. doi: 10.1016/j.cbi.2023.110838. Epub 2023 Dec 16. Chem Biol Interact. 2024. PMID: 38104745 Review.
-
Cilastatin as a protective agent against drug-induced nephrotoxicity: a literature review.Expert Opin Drug Saf. 2020 Aug;19(8):999-1010. doi: 10.1080/14740338.2020.1796967. Epub 2020 Jul 23. Expert Opin Drug Saf. 2020. PMID: 32666842 Review.
References
-
- Drug-induced nephrotoxicity: pathogenic mechanisms, biomarkers and prevention strategies. Wu H, Huang J. Curr Drug Metab. 2018;19:559–567. - PubMed
-
- Drug-induced acute kidney injury: diverse mechanisms of tubular injury. Perazella MA. Curr Opin Crit Care. 2019;25:550–557. - PubMed
-
- Dipeptidyl peptidase-4 inhibitor teneligliptin accelerates recovery from cisplatin-induced acute kidney injury by attenuating inflammation and promoting tubular regeneration. Iwakura T, Zhao Z, Marschner JA, Devarapu SK, Yasuda H, Anders HJ. Nephrol Dial Transplant. 2019;34:1669–1680. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
