Comparison of the structure and function of a chimeric peptide modified titanium surface
- PMID: 35530988
- PMCID: PMC9070349
- DOI: 10.1039/c9ra05127a
Comparison of the structure and function of a chimeric peptide modified titanium surface
Abstract
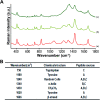
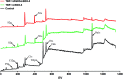
Peri-implantitis is a plaque-initiating infectious disease that can be prevented by interfering with the initial bacterial attachment. At present, surface modification of implants using antimicrobial peptides can interfere with the adhesion of streptococci. In this study, the structure and function of chimeric peptides were compared to get a strategy to modify a Ti surface. Compared to the antimicrobial activity with a fragment of hBD-3, the bifunctional and multifunctional chimeric peptides retain their antimicrobial function. All peptides showed antimicrobial activity against streptococcus in biofilm and planktonic conditions. The results demonstrate significant improvement in reducing bacterial colonization onto titanium surfaces. According to the results of structure analysis, the antimicrobial activity of tyrosine in hBD3-3 was stronger than that of the alpha helix in bifunctional or multifunctional chimeric peptides. Rigid connections were proved to avoid functional domain changes due to the interaction of charges. These results indicated that the endogenous peptide fragments modifying the Ti surface could provide an environmentally friendly approach to reduce or prevent the occurrence of peri-implant diseases.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest relating to this work.
Figures

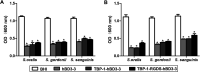

Similar articles
-
Engineered chimeric peptides with antimicrobial and titanium-binding functions to inhibit biofilm formation on Ti implants.Mater Sci Eng C Mater Biol Appl. 2018 Jan 1;82:141-154. doi: 10.1016/j.msec.2017.08.062. Epub 2017 Aug 18. Mater Sci Eng C Mater Biol Appl. 2018. PMID: 29025642
-
Modification of Titanium Substrates with Chimeric Peptides Comprising Antimicrobial and Titanium-Binding Motifs Connected by Linkers To Inhibit Biofilm Formation.ACS Appl Mater Interfaces. 2016 Mar 2;8(8):5124-36. doi: 10.1021/acsami.5b11949. Epub 2016 Feb 22. ACS Appl Mater Interfaces. 2016. PMID: 26863404
-
Modification of the surface of titanium with multifunctional chimeric peptides to prevent biofilm formation via inhibition of initial colonizers.Int J Nanomedicine. 2018 Sep 12;13:5361-5375. doi: 10.2147/IJN.S170819. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30254440 Free PMC article.
-
Bioactive coatings for dental implants: A review of alternative strategies to prevent peri-implantitis induced by anaerobic bacteria.Anaerobe. 2021 Aug;70:102404. doi: 10.1016/j.anaerobe.2021.102404. Epub 2021 Jun 17. Anaerobe. 2021. PMID: 34146701 Review.
-
Emerging antibacterial biomaterial strategies for the prevention of peri-implant inflammatory diseases.Int J Oral Maxillofac Implants. 2011 May-Jun;26(3):553-60. Int J Oral Maxillofac Implants. 2011. PMID: 21691602 Review.
Cited by
-
Biomaterials science and surface engineering strategies for dental peri-implantitis management.Mil Med Res. 2024 May 13;11(1):29. doi: 10.1186/s40779-024-00532-9. Mil Med Res. 2024. PMID: 38741175 Free PMC article. Review.
-
Application of Antimicrobial Peptides on Biomedical Implants: Three Ways to Pursue Peptide Coatings.Int J Mol Sci. 2021 Dec 8;22(24):13212. doi: 10.3390/ijms222413212. Int J Mol Sci. 2021. PMID: 34948009 Free PMC article. Review.
-
Immobilised antimicrobial peptides in downregulation of biofilm.Naunyn Schmiedebergs Arch Pharmacol. 2024 Aug;397(8):5559-5569. doi: 10.1007/s00210-024-03056-0. Epub 2024 Mar 27. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38536433 Review.
References
-
- Berglundh T. Armitage G. Araujo M. G. Avila-Ortiz G. Blanco J. Camargo P. M. Chen S. Cochran D. Derks J. Figuero E. Hammerle C. H. F. Heitz-Mayfield L. J. A. Huynh-Ba G. Iacono V. Koo K. T. Lambert F. McCauley L. Quirynen M. Renvert S. Salvi G. E. Schwarz F. Tarnow D. Tomasi C. Wang H. L. Zitzmann N. J. Periodontol. 2018;89(1):S313–S318. doi: 10.1002/JPER.17-0739. - DOI - PubMed
LinkOut - more resources
Full Text Sources

