A catalyst- and solvent-free protocol for the sustainable synthesis of fused 4 H-pyran derivatives
- PMID: 35531009
- PMCID: PMC9070424
- DOI: 10.1039/c9ra04370e
A catalyst- and solvent-free protocol for the sustainable synthesis of fused 4 H-pyran derivatives
Abstract
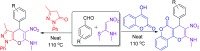
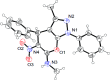
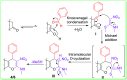
An efficient and cost-effective method was developed for the synthesis of two kinds of fused 4H-pyran derivatives, namely, dihydropyrano[2,3-c]pyrazole 4 and pyrano[3,2-c]chromenone 6. The reactions of 3-methyl-1-phenyl-5-pyrazolone/4-hydroxycoumarin with aromatic aldehydes and (E)-N-methyl-1-(methylthio)-2-nitroethenamine (NMSM), involving the Knoevenagel, Michael-addition, O-cyclization and elimination reactions under thermal heating, afforded the desired products. The synthesized compounds were characterized by standard spectroscopic techniques. Further, the structures of pyrazole-fused 4H-pyran 4a and coumarin-fused 4H-pyran 6b were confirmed by single-crystal XRD analysis. The short reaction time, good-to-excellent yields, elimination of the use of expensive, metallic and toxic catalysts or hazardous organic solvents and high atom-economy are some noteworthy features of this protocol.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Anastas P. T. and Warner J. C., Green Chemistry: Theory and Practice, Oxford University Press Inc., 1st edn, New York, 1998
- Kirchhoff M. M. Environ. Sci. Technol. 2003;37:5349. doi: 10.1021/es0346072. - DOI - PubMed
- Horvath I. T. Anastas P. T. Chem. Rev. 2007;107:2169. doi: 10.1021/cr078380v. - DOI - PubMed
-
- Khan M. M. Khan S. Saigal Iqbal S. RSC Adv. 2016;6:42045. doi: 10.1039/C6RA06767K. - DOI
- Khan A. T. Das D. K. Khan M. M. Tetrahedron Lett. 2011;52:4539. doi: 10.1016/j.tetlet.2011.06.080. - DOI
- Cioc R. C. Ruijter E. Orru R. V. A. Green Chem. 2014;16:2958. doi: 10.1039/C4GC00013G. - DOI
- Khan A. T. Khan M. M. Das D. K. Lal M. J. Heterocycl. Chem. 2012;49:1362. doi: 10.1002/jhet.1017. - DOI
-
- Alvim H. G. O. Correa J. R. Assumpcao J. A. F. da Silva W. A. Rodrigues M. O. de Macedo J. L. Fioramonte M. Gozzo F. C. Gatt C. C. Neto B. A. D. J. Org. Chem. 2018;83:4044. doi: 10.1021/acs.joc.8b00472. - DOI - PubMed
- Alvim H. G. O. Pinheiro D. L. J. Carvalho-Silva V. H. Fioramonte M. Gozzo F. C. da Silva W. A. Amarante G. W. Neto B. A. D. J. Org. Chem. 2018;83:12143. doi: 10.1021/acs.joc.8b02101. - DOI - PubMed
- Zeng L. Huang B. Shen Y. Cui S. Org. Lett. 2018;20:3460. doi: 10.1021/acs.orglett.8b01159. - DOI - PubMed
LinkOut - more resources
Full Text Sources

