Ramified derivatives of 5-(perylen-3-ylethynyl)uracil-1-acetic acid and their antiviral properties
- PMID: 35531032
- PMCID: PMC9070374
- DOI: 10.1039/c9ra06313g
Ramified derivatives of 5-(perylen-3-ylethynyl)uracil-1-acetic acid and their antiviral properties
Abstract
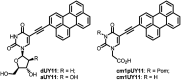
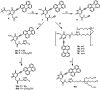
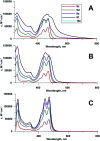
The propargylamide of N3-Pom-protected 5-(perylen-3-ylethynyl)uracil acetic acid, a universal precursor, was used in a CuAAC click reaction for the synthesis of several derivatives, including three ramified molecules with high activities against tick-borne encephalitis virus (TBEV). Pentaerythritol-based polyazides were used for the assembly of molecules containing 2⋯4 antiviral 5-(perylen-3-ylethynyl)uracil scaffolds, the first examples of polyvalent perylene antivirals. Cluster compounds showed enhanced absorbance, however, their fluorescence was reduced due to self-quenching. Due to the solubility issues, Pom group removal succeeded only for compounds with one peryleneethynyluracil unit. Four compounds, including one ramified cluster 9f, showed remarkable 1⋯3 nM EC50 values against TBEV in cell culture.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures
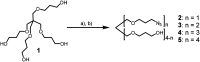

References
-
- Korshun V. A. Manasova E. V. Balakin K. V. Malakhov A. D. Perepelov A. V. Sokolova T. A. Berlin Y. A. Nucleosides Nucleotides. 1998;17:1809–1812. doi: 10.1080/07328319808004718. - DOI
- Skorobogatyi M. V. Malakhov A. D. Pchelintseva A. A. Turban A. A. Bondarev S. L. Korshun V. A. ChemBioChem. 2006;7:810–816. doi: 10.1002/cbic.200600040. - DOI - PubMed
-
- Andronova V. L. Skorobogatyi M. V. Manasova E. V. Berlin Y. A. Korshun V. A. Galegov G. A. Russ. J. Bioorg. Chem. 2003;29:262–266. doi: 10.1023/A:1023936516589. - DOI - PubMed
- Skorobogatyi M. V. Ustinov A. V. Stepanova I. A. Pchelintseva A. A. Petrunina A. L. Andronova V. L. Galegov G. A. Malakhov A. D. Korshun V. A. Org. Biomol. Chem. 2006;4:1091–1096. doi: 10.1039/B516804J. - DOI - PubMed
- St. Vincent M. R. Colpitts C. C. Ustinov A. V. Muqadas M. Joyce M. A. Barsby N. A. Epand R. F. Epand R. M. Khramyshev S. A. Valueva O. A. Korshun V. A. Tyrrell D. L. J. Schang L. M. Proc. Natl. Acad. Sci. U. S. A. 2010;107:17339–17344. doi: 10.1073/pnas.1010026107. - DOI - PMC - PubMed
- Orlov A. A. Chistov A. A. Kozlovskaya L. I. Ustinov A. V. Korshun V. A. Karganova G. G. Osolodkin D. I. Med. Chem. Commun. 2016;7:495–499. doi: 10.1039/C5MD00538H. - DOI
-
- Schang L. M. Future Virol. 2014;9:283–299. doi: 10.2217/fvl.13.130. - DOI
LinkOut - more resources
Full Text Sources

