Condensed Mitochondria Assemble Into the Acrosomal Matrix During Spermiogenesis
- PMID: 35531097
- PMCID: PMC9068883
- DOI: 10.3389/fcell.2022.867175
Condensed Mitochondria Assemble Into the Acrosomal Matrix During Spermiogenesis
Abstract
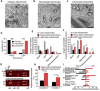
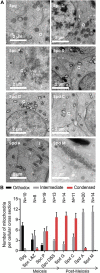
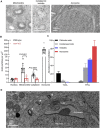
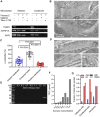
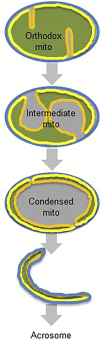
Mammalian spermatogenesis is associated with the transient appearance of condensed mitochondria, a singularity of germ cells with unknown function. Using proteomic analysis, respirometry, and electron microscopy with tomography, we studied the development of condensed mitochondria. Condensed mitochondria arose from orthodox mitochondria during meiosis by progressive contraction of the matrix space, which was accompanied by an initial expansion and a subsequent reduction of the surface area of the inner membrane. Compared to orthodox mitochondria, condensed mitochondria respired more actively, had a higher concentration of respiratory enzymes and supercomplexes, and contained more proteins involved in protein import and expression. After the completion of meiosis, the abundance of condensed mitochondria declined, which coincided with the onset of the biogenesis of acrosomes. Immuno-electron microscopy and the analysis of sub-cellular fractions suggested that condensed mitochondria or their fragments were translocated into the lumen of the acrosome. Thus, it seems condensed mitochondria are formed from orthodox mitochondria by extensive transformations in order to support the formation of the acrosomal matrix.
Keywords: acrosome; cristae; mitochondria; spermatogenesis; spermiogenensis.
Copyright © 2022 Ren, Xu, Phoon, Erdjument-Bromage, Neubert, Rajan, Hussain and Schlame.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Inhibition of autophagy impairs acrosome and mitochondrial crista formation during spermiogenesis in turtle: Ultrastructural evidence.Micron. 2019 Jun;121:84-89. doi: 10.1016/j.micron.2019.03.006. Epub 2019 Apr 1. Micron. 2019. PMID: 30953869
-
Mitochondrial differentiation during meiosis of male germ cells.Int J Androl. 1995 Dec;18 Suppl 2:7-11. Int J Androl. 1995. PMID: 8719852
-
Spermatogenesis revisited. IV. Abnormal spermiogenesis in mice homozygous for another male-sterility-inducing mutation, hpy (hydrocephalic-polydactyl).Cell Tissue Res. 1977 May 16;180(2):187-201. doi: 10.1007/BF00231951. Cell Tissue Res. 1977. PMID: 872193
-
The role of the acrosomal matrix in fertilization.Int J Dev Biol. 2008;52(5-6):511-22. doi: 10.1387/ijdb.072532mb. Int J Dev Biol. 2008. PMID: 18649264 Review.
-
Organization and modifications of sperm acrosomal molecules during spermatogenesis and epididymal maturation.Microsc Res Tech. 2003 May 1;61(1):39-45. doi: 10.1002/jemt.10315. Microsc Res Tech. 2003. PMID: 12672121 Review.
Cited by
-
The critical role of cardiolipin in metazoan differentiation, development, and maturation.Dev Dyn. 2023 Jun;252(6):691-712. doi: 10.1002/dvdy.567. Epub 2023 Feb 9. Dev Dyn. 2023. PMID: 36692477 Free PMC article. Review.
-
Dnajc5b contributes to male fertility by maintaining the mitochondrial functions and autophagic homeostasis during spermiogenesis.Cell Mol Life Sci. 2025 Feb 3;82(1):69. doi: 10.1007/s00018-024-05552-1. Cell Mol Life Sci. 2025. PMID: 39899042 Free PMC article.
-
Inhibition of Mitochondrial Uncoupling Proteins Arrests Human Spermatozoa Motility without Compromising Viability.Antioxidants (Basel). 2023 Feb 8;12(2):409. doi: 10.3390/antiox12020409. Antioxidants (Basel). 2023. PMID: 36829970 Free PMC article.
-
MicroRNAs and Their Associated Genes Regulating the Acrosome Reaction in Sperm of High- versus Low-Fertility Holstein Bulls.Animals (Basel). 2024 Mar 8;14(6):833. doi: 10.3390/ani14060833. Animals (Basel). 2024. PMID: 38539931 Free PMC article.
-
Mitochondrial epigenetics brings new perspectives on doubly uniparental inheritance in bivalves.Sci Rep. 2024 Dec 28;14(1):31544. doi: 10.1038/s41598-024-83368-6. Sci Rep. 2024. PMID: 39733193 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

