Retinoic Acid is Required for Normal Morphogenetic Movements During Gastrulation
- PMID: 35531100
- PMCID: PMC9068879
- DOI: 10.3389/fcell.2022.857230
Retinoic Acid is Required for Normal Morphogenetic Movements During Gastrulation
Abstract
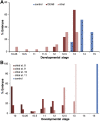
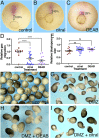
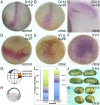
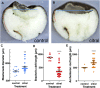
Retinoic acid (RA) is a central regulatory signal that controls numerous developmental processes in vertebrate embryos. Although activation of Hox expression is considered one of the earliest functions of RA signaling in the embryo, there is evidence that embryos are poised to initiate RA signaling just before gastrulation begins, and manipulations of the RA pathway have been reported to show gastrulation defects. However, which aspects of gastrulation are affected have not been explored in detail. We previously showed that partial inhibition of RA biosynthesis causes a delay in the rostral migration of some of the earliest involuting cells, the leading edge mesendoderm (LEM) and the prechordal mesoderm (PCM). Here we identify several detrimental gastrulation defects resulting from inhibiting RA biosynthesis by three different treatments. RA reduction causes a delay in the progression through gastrulation as well as the rostral migration of the goosecoid-positive PCM cells. RA inhibition also hampered the elongation of explanted dorsal marginal zones, the compaction of the blastocoel, and the length of Brachet's cleft, all of which indicate an effect on LEM/PCM migration. The cellular mechanisms underlying this deficit were shown to include a reduced deposition of fibronectin along Brachet's cleft, the substrate for their migration, as well as impaired separation of the blastocoel roof and involuting mesoderm, which is important for the formation of Brachet's cleft and successful LEM/PCM migration. We further show reduced non-canonical Wnt signaling activity and altered expression of genes in the Ephrin and PDGF signaling pathways, both of which are required for the rostral migration of the LEM/PCM, following RA reduction. Together, these experiments demonstrate that RA signaling performs a very early function critical for the progression of gastrulation morphogenetic movements.
Keywords: Brachet’s cleft; Xenopus embryo; embryo development; gastrulation delay; morphogenetic movements; retinoic acid signaling; tissue separation.
Copyright © 2022 Gur, Edri, Moody and Fainsod.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Brachet's cleft: a model for the analysis of tissue separation in Xenopus.Wiley Interdiscip Rev Dev Biol. 2012 Mar-Apr;1(2):294-300. doi: 10.1002/wdev.24. Epub 2011 Dec 14. Wiley Interdiscip Rev Dev Biol. 2012. PMID: 23801443 Review.
-
Mechanisms of mesendoderm internalization in the Xenopus gastrula: lessons from the ventral side.Dev Biol. 2001 Dec 1;240(1):108-22. doi: 10.1006/dbio.2001.0459. Dev Biol. 2001. PMID: 11784050
-
Directional migration of leading-edge mesoderm generates physical forces: Implication in Xenopus notochord formation during gastrulation.Dev Biol. 2013 Oct 15;382(2):482-95. doi: 10.1016/j.ydbio.2013.07.023. Epub 2013 Aug 6. Dev Biol. 2013. PMID: 23933171
-
Roles for Xenopus aquaporin-3b (aqp3.L) during gastrulation: Fibrillar fibronectin and tissue boundary establishment in the dorsal margin.Dev Biol. 2018 Jan 1;433(1):3-16. doi: 10.1016/j.ydbio.2017.11.001. Epub 2017 Nov 4. Dev Biol. 2018. PMID: 29113748
-
Internalizing the vegetal cell mass before and during amphibian gastrulation: vegetal rotation and related movements.Wiley Interdiscip Rev Dev Biol. 2012 Mar-Apr;1(2):301-6. doi: 10.1002/wdev.26. Epub 2011 Dec 27. Wiley Interdiscip Rev Dev Biol. 2012. PMID: 23801444 Review.
Cited by
-
Essential Oils Produce Developmental Toxicity in Zebrafish Embryos and Cause Behavior Changes in Zebrafish Larvae.Biomedicines. 2023 Oct 18;11(10):2821. doi: 10.3390/biomedicines11102821. Biomedicines. 2023. PMID: 37893194 Free PMC article.
-
Alcohol induces neural tube defects by reducing retinoic acid signaling and promoting neural plate expansion.Front Cell Dev Biol. 2023 Dec 5;11:1282273. doi: 10.3389/fcell.2023.1282273. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38116205 Free PMC article.
-
Genetically programmed retinoic acid deficiency during gastrulation phenocopies most known developmental defects due to acute prenatal alcohol exposure in FASD.Front Cell Dev Biol. 2023 Jun 16;11:1208279. doi: 10.3389/fcell.2023.1208279. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37397253 Free PMC article.
-
Developmental system drift and modular gene regulatory networks shape gastrulation in Acropora.Life Sci Alliance. 2025 Aug 14;8(11):e202503293. doi: 10.26508/lsa.202503293. Print 2025 Nov. Life Sci Alliance. 2025. PMID: 40813231 Free PMC article.
References
LinkOut - more resources
Full Text Sources

