A simple LC-MS/MS method for pharmacokinetic study of carvedilol and 4/-hydroxyphenyl carvedilol at a low dose
- PMID: 35531138
- PMCID: PMC9075024
- DOI: 10.4103/1735-5362.343077
A simple LC-MS/MS method for pharmacokinetic study of carvedilol and 4/-hydroxyphenyl carvedilol at a low dose
Abstract
Background and purpose: The study was aimed at validating a simple, rapid, and low-cost LC-MS/MS method for carvedilol and 4/-hydroxyphenyl carvedilol assay in human plasma. The validated method was applied to investigate the pharmacokinetics after a low dose of 6.25 mg. carvedilol.
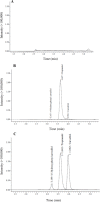
Experimental approach: In this study, the plasma was extracted by liquid-liquid extraction and evaporated the organic layer to dryness, then both analytes in the residue were reconstituted and detected by LC- MS/MS. The method was validated following the guideline on bioanalytical method validation. Thirty-one healthy volunteers participated in the pharmacokinetic study. After 10 h of fasting, each volunteer received one tablet of 6.25 mg carvedilol orally. Blood samples were collected at 16 prescheduled time points. The plasma samples were analyzed for pharmacokinetics.
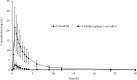
Findings/results: The method was linear over a range of 0.050-50.049 ng/mL for carvedilol and 0.050- 10.017 ng/mL for 4/-hydroxyphenyl carvedilol. Crucial validated results reached the requirements of selectivity, accuracy, precision, and stability. Pharmacokinetics of carvedilol and 4/-hydroxyphenyl carvedilol were evaluated which showed Cmax at 21.26 ± 9.23 and 2.42 ± 2.07 ng/mL; AUC0-t 66.95 ± 29.45 and 5.93 ± 3.51 ng.h/mL; AUC0-inf 68.54 ± 30.11 and 6.78 ± 3.49 ng.h/mL; and T1/2 6.30 ± 1.95 and 6.31 ± 6.45 h, respectively.
Conclusion and implications: The validated method was able to detect and quantify both analytes in plasma samples and can be applied to the pharmacokinetic study of carvedilol and 4/-hydroxyphenyl carvedilol after receiving carvedilol at 6.25 mg orally.
Keywords: 4/-Hydroxyphenyl carvedilol; Carvedilol; Pharmacokinetics; Tandem mass spectrometry.
Copyright: © 2022 Research in Pharmaceutical Sciences.
Conflict of interest statement
The authors declared no conflicts of interest in this study.
Figures
Similar articles
-
A validated enantioselective LC-MS/MS assay for the simultaneous determination of carvedilol and its pharmacologically active 4'-hydroxyphenyl metabolite in human plasma: application to a clinical pharmacokinetic study.J Pharm Biomed Anal. 2012 Nov;70:574-9. doi: 10.1016/j.jpba.2012.05.026. Epub 2012 May 30. J Pharm Biomed Anal. 2012. PMID: 22709607 Clinical Trial.
-
UPLC-MS/MS assay for the simultaneous quantification of carvedilol and its active metabolite 4'-hydroxyphenyl carvedilol in human plasma to support a bioequivalence study in healthy volunteers.Biomed Chromatogr. 2013 Aug;27(8):974-86. doi: 10.1002/bmc.2889. Epub 2013 Mar 11. Biomed Chromatogr. 2013. PMID: 23483571 Clinical Trial.
-
Low-volume LC-MS/MS method for the pharmacokinetic investigation of carvedilol, enalapril and their metabolites in whole blood and plasma: Application to a paediatric clinical trial.Drug Test Anal. 2021 Mar;13(3):694-708. doi: 10.1002/dta.2949. Epub 2020 Nov 22. Drug Test Anal. 2021. PMID: 33126289
-
Simultaneous quantification of vortioxetine, carvedilol and its active metabolite 4-hydroxyphenyl carvedilol in rat plasma by UPLC-MS/MS: Application to their pharmacokinetic interaction study.J Pharm Biomed Anal. 2016 Sep 5;128:184-190. doi: 10.1016/j.jpba.2016.05.029. Epub 2016 May 19. J Pharm Biomed Anal. 2016. PMID: 27262994
-
Simultaneous quantification of carvedilol and its metabolites in rat plasma by ultra performance liquid chromatography tandem mass spectrometry and pharmacokinetic application.J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Jan 1;974:138-46. doi: 10.1016/j.jchromb.2014.10.037. Epub 2014 Nov 4. J Chromatogr B Analyt Technol Biomed Life Sci. 2015. PMID: 25463209
Cited by
-
In Vitro Permeation Studies on Carvedilol Containing Dissolving Microarray Patches Quantified Using a Rapid and Simple HPLC-UV Analytical Method.AAPS PharmSciTech. 2022 Oct 4;23(7):273. doi: 10.1208/s12249-022-02422-6. AAPS PharmSciTech. 2022. PMID: 36195761
References
-
- McTavish D, Campoli-Richards D, Sorkin EM. Carvedilol: a review of its pharmacodynamics and pharmacokinetic properties, and therapeutic efficacy. Drugs. 1993;45(2):232–258. DOI: 10.2165/00003495-199345020-00006. - PubMed
-
- Keating GM, Jarvis B. Carvedilol: a review of its use in chronic heart failure. Drugs. 2003;63(16):1697–1741. DOI: 10.2165/00003495-200363160-00006. - PubMed
-
- Morgan T, Anderson A, Cripps J, Adam W. Pharmacokinetics of carvedilol in older and younger patients. J Hum Hypertens. 1990;4(6):709–715. PMID: 2096213. - PubMed
-
- Mollendorff E, Reiff K, Neugebauer G. Pharmacokinetics and bioavailability of carvedilol, a vasodilating beta-blocker. Eur J Clin Pharmacol. 1987;33(5):511–513. DOI: 10.1007/BF00544245. - PubMed
LinkOut - more resources
Full Text Sources
