F1-ATPase Rotary Mechanism: Interpreting Results of Diverse Experimental Modes With an Elastic Coupling Theory
- PMID: 35531282
- PMCID: PMC9072658
- DOI: 10.3389/fmicb.2022.861855
F1-ATPase Rotary Mechanism: Interpreting Results of Diverse Experimental Modes With an Elastic Coupling Theory
Abstract
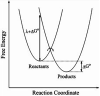
In this chapter, we review single-molecule observations of rotary motors, focusing on the general theme that their mechanical motion proceeds in substeps with each substep described by an angle-dependent rate constant. In the molecular machine F1-ATPase, the stepping rotation is described for individual steps by forward and back reaction rate constants, some of which depend strongly on the rotation angle. The rotation of a central shaft is typically monitored by an optical probe. We review our recent work on the theory for the angle-dependent rate constants built to treat a variety of single-molecule and ensemble experiments on the F1-ATPase, and relating the free energy of activation of a step to the standard free energy of reaction for that step. This theory, an elastic molecular transfer theory, provides a framework for a multistate model and includes the probe used in single-molecule imaging and magnetic manipulation experiments. Several examples of its application are the following: (a) treatment of the angle-dependent rate constants in stalling experiments, (b) use of the model to enhance the time resolution of the single-molecule imaging apparatus and to detect short-lived states with a microsecond lifetime, states hidden by the fluctuations of the imaging probe, (c) treatment of out-of-equilibrium "controlled rotation" experiments, (d) use of the model to predict, without adjustable parameters, the angle-dependent rate constants of nucleotide binding and release, using data from other experiments, and (e) insights obtained from correlation of kinetic and cryo-EM structural data. It is also noted that in the case where the release of ADP would be a bottleneck process, the binding of ATP to another site acts to accelerate the release by 5-6 orders of magnitude. The relation of the present set of studies to previous and current theoretical work in the field is described. An overall goal is to gain mechanistic insight into the biological function in relation to structure.
Keywords: ATP binding; F1-ATPase; concerted kinetics; cryo-electron microscopy; multi-state theory; rotary biomolecular motors; single-molecule imaging; stepping rotation of F1-ATPase.
Copyright © 2022 Volkán-Kacsó and Marcus.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

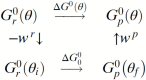
Similar articles
-
Theory of single-molecule controlled rotation experiments, predictions, tests, and comparison with stalling experiments in F1-ATPase.Proc Natl Acad Sci U S A. 2016 Oct 25;113(43):12029-12034. doi: 10.1073/pnas.1611601113. Epub 2016 Oct 10. Proc Natl Acad Sci U S A. 2016. PMID: 27790985 Free PMC article.
-
Single-molecule analysis reveals rotational substeps and chemo-mechanical coupling scheme of Enterococcus hirae V1-ATPase.J Biol Chem. 2019 Nov 8;294(45):17017-17030. doi: 10.1074/jbc.RA119.008947. Epub 2019 Sep 13. J Biol Chem. 2019. PMID: 31519751 Free PMC article.
-
Angle-dependent rotation velocity consistent with ADP release in bacterial F1-ATPase.Front Mol Biosci. 2023 Aug 2;10:1184249. doi: 10.3389/fmolb.2023.1184249. eCollection 2023. Front Mol Biosci. 2023. PMID: 37602322 Free PMC article.
-
How Does F1-ATPase Generate Torque?: Analysis From Cryo-Electron Microscopy and Rotational Catalysis of Thermophilic F1.Front Microbiol. 2022 May 6;13:904084. doi: 10.3389/fmicb.2022.904084. eCollection 2022. Front Microbiol. 2022. PMID: 35602057 Free PMC article. Review.
-
Insights into the mechanism of ATP-driven rotary motors from direct torque measurement.Biophys Rev. 2019 Aug;11(4):653-657. doi: 10.1007/s12551-019-00564-9. Epub 2019 Jul 18. Biophys Rev. 2019. PMID: 31321734 Free PMC article. Review.
Cited by
-
2-Site versus 3-site models of ATP hydrolysis by F1-ATPase: definitive mathematical proof using combinatorics and conservation equations.Theory Biosci. 2024 Sep;143(3):217-227. doi: 10.1007/s12064-024-00421-8. Epub 2024 Jul 30. Theory Biosci. 2024. PMID: 39078560
-
Beyond binding change: the molecular mechanism of ATP hydrolysis by F1-ATPase and its biochemical consequences.Front Chem. 2023 May 30;11:1058500. doi: 10.3389/fchem.2023.1058500. eCollection 2023. Front Chem. 2023. PMID: 37324562 Free PMC article.
References
-
- Agmon N., Hopfield J. J. (1983). Transient kinetics of chemical reactions with bounded diffusion perpendicular to the reaction coordinate: Intramolecular processes with slow conformational changes. J. Chem. Phys. 78, 6947–6959. doi: 10.1063/1.444643 - DOI
Publication types
LinkOut - more resources
Full Text Sources

