A Cecropin-4 Derived Peptide C18 Inhibits Candida albicans by Disturbing Mitochondrial Function
- PMID: 35531288
- PMCID: PMC9075107
- DOI: 10.3389/fmicb.2022.872322
A Cecropin-4 Derived Peptide C18 Inhibits Candida albicans by Disturbing Mitochondrial Function
Abstract
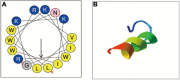
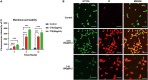
Global burden of fungal infections and related health risk has accelerated at an incredible pace, and multidrug resistance emergency aggravates the need for the development of new effective strategies. Candida albicans is clinically the most ubiquitous pathogenic fungus that leads to high incidence and mortality in immunocompromised patients. Antimicrobial peptides (AMPs), in this context, represent promising alternatives having potential to be exploited for improving human health. In our previous studies, a Cecropin-4-derived peptide named C18 was found to possess a broader antibacterial spectrum after modification and exhibit significant antifungal activity against C. albicans. In this study, C18 shows antifungal activity against C. albicans or non-albicans Candida species with a minimum inhibitory concentration (MIC) at 4∼32 μg/ml, and clinical isolates of fluconazole (FLZ)-resistance C. tropicalis were highly susceptible to C18 with MIC value of 8 or 16 μg/ml. Additionally, C18 is superior to FLZ for killing planktonic C. albicans from inhibitory and killing kinetic curves. Moreover, C18 could attenuate the virulence of C. albicans, which includes damaging the cell structure, retarding hyphae transition, and inhibiting biofilm formation. Intriguingly, in the Galleria mellonella model with C. albicans infection, C18 could improve the survival rate of G. mellonella larvae to 70% and reduce C. albicans load from 5.01 × 107 to 5.62 × 104 CFU. For mechanistic action of C18, the level of reactive oxygen species (ROS) generation and cytosolic Ca2 + increased in the presence of C18, which is closely associated with mitochondrial dysfunction. Meanwhile, mitochondrial membrane potential (△Ψm) loss and ATP depletion of C. albicans occurred with the treatment of C18. We hypothesized that C18 might inhibit C. albicans via triggering mitochondrial dysfunction driven by ROS generation and Ca2 + accumulation. Our observation provides a basis for future research to explore the antifungal strategies and presents C18 as an attractive therapeutic candidate to be developed to treat candidiasis.
Keywords: Candida albicans; G. mellonella; ROS; antifungal activity; cecropin-4 derived peptide; mitochondrial dysfunction.
Copyright © 2022 Sun, Peng, Yang, Jiao, Zhou, Tao, Zhu, Tian, Huang and Guo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
Mechanisms of Action of the Antimicrobial Peptide Cecropin in the Killing of Candida albicans.Life (Basel). 2022 Oct 11;12(10):1581. doi: 10.3390/life12101581. Life (Basel). 2022. PMID: 36295016 Free PMC article.
-
WMR Peptide as Antifungal and Antibiofilm against Albicans and Non-Albicans Candida Species: Shreds of Evidence on the Mechanism of Action.Int J Mol Sci. 2022 Feb 15;23(4):2151. doi: 10.3390/ijms23042151. Int J Mol Sci. 2022. PMID: 35216270 Free PMC article.
-
Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis.J Mycol Med. 2020 Apr;30(1):100911. doi: 10.1016/j.mycmed.2019.100911. Epub 2019 Nov 7. J Mycol Med. 2020. PMID: 32008964
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
-
Resistance in human pathogenic yeasts and filamentous fungi: prevalence, underlying molecular mechanisms and link to the use of antifungals in humans and the environment.Dan Med J. 2016 Oct;63(10):B5288. Dan Med J. 2016. PMID: 27697142 Review.
Cited by
-
The Antimicrobial Peptide AMP-17 Derived from Musca domestica Inhibits Biofilm Formation and Eradicates Mature Biofilm in Candida albicans.Antibiotics (Basel). 2022 Oct 25;11(11):1474. doi: 10.3390/antibiotics11111474. Antibiotics (Basel). 2022. PMID: 36358129 Free PMC article.
-
Appressoria Formation in Phytopathogenic Fungi Suppressed by Antimicrobial Peptides and Hybrid Peptides from Black Soldier Flies.Genes (Basel). 2023 May 17;14(5):1096. doi: 10.3390/genes14051096. Genes (Basel). 2023. PMID: 37239456 Free PMC article.
-
The Antifungal Effects of Equol Against Candida albicans Involve Mitochondrial Dysfunction.J Fungi (Basel). 2025 Apr 27;11(5):339. doi: 10.3390/jof11050339. J Fungi (Basel). 2025. PMID: 40422673 Free PMC article.
-
Cec4-Derived Peptide Inhibits Planktonic and Biofilm-Associated Methicillin Resistant Staphylococcus epidermidis.Microbiol Spectr. 2022 Dec 21;10(6):e0240922. doi: 10.1128/spectrum.02409-22. Epub 2022 Dec 1. Microbiol Spectr. 2022. PMID: 36453944 Free PMC article.
-
[Low-intensity pulsed ultrasound combined with nystatin treatment synergistically inhibits vaginal Candida albicans biofilm infection in rabbits].Nan Fang Yi Ke Da Xue Xue Bao. 2025 Feb 20;45(2):296-303. doi: 10.12122/j.issn.1673-4254.2025.02.10. Nan Fang Yi Ke Da Xue Xue Bao. 2025. PMID: 40031973 Free PMC article. Chinese.
References
-
- Aguiar F. L. L., Santos N. C., de Paula Cavalcante C. S., Andreu D., Baptista G. R., Gonçalves S. (2020). Antibiofilm activity on candida albicans and mechanism of action on biomembrane models of the antimicrobial peptide ctn[15-34]. Int. J. Mol. Sci. 21:339. 10.3390/ijms21218339 - DOI - PMC - PubMed
-
- Biernasiuk A., Berecka-Rycerz A., Gumieniczek A., Malm M., Łączkowski K. Z., Szymańska J., et al. (2021). The newly synthesized thiazole derivatives as potential antifungal compounds against Candida albicans. Appl. Microbiol. Biotechnol. 105 6355–6367. 10.1007/s00253-021-11477-7 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

