Community Assembly and Stability in the Root Microbiota During Early Plant Development
- PMID: 35531294
- PMCID: PMC9069014
- DOI: 10.3389/fmicb.2022.826521
Community Assembly and Stability in the Root Microbiota During Early Plant Development
Abstract
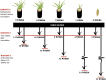
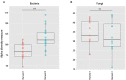
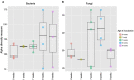
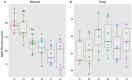
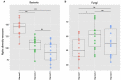
Little is known about how community composition in the plant microbiome is affected by events in the life of a plant. For example, when the plant is exposed to soil, microbial communities may be an important factor in root community assembly. We conducted two experiments asking whether the composition of the root microbiota in mature plants could be determined by either the timing of root exposure to microbial communities or priority effects by early colonizing microbes. Timing of microbial exposure was manipulated through an inoculation experiment, where plants of different ages were exposed to a common soil inoculum. Priority effects were manipulated by challenging roots with established microbiota with an exogenous microbial community. Results show that even plants with existing microbial root communities were able to acquire new microbial associates, but that timing of soil exposure affected root microbiota composition for both bacterial and fungal communities in mature plants. Plants already colonized were only receptive to colonizers at 1 week post-germination. Our study shows that the timing of soil exposure in the early life stages of a plant is important for the development of the root microbiota in mature plants.
Keywords: Setaria viridis; bacteria; fungi; plant microbiome; priority effects; root microbiota; soil exposure.
Copyright © 2022 Aleklett, Rosa, Pickles and Hart.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
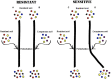




Similar articles
-
Maize Field Study Reveals Covaried Microbiota and Metabolic Changes in Roots over Plant Growth.mBio. 2022 Apr 26;13(2):e0258421. doi: 10.1128/mbio.02584-21. Epub 2022 Mar 8. mBio. 2022. PMID: 35258335 Free PMC article.
-
Lotus japonicus Symbiosis Genes Impact Microbial Interactions between Symbionts and Multikingdom Commensal Communities.mBio. 2019 Oct 8;10(5):e01833-19. doi: 10.1128/mBio.01833-19. mBio. 2019. PMID: 31594815 Free PMC article.
-
Maize synthesized benzoxazinoids affect the host associated microbiome.Microbiome. 2019 Apr 11;7(1):59. doi: 10.1186/s40168-019-0677-7. Microbiome. 2019. PMID: 30975184 Free PMC article.
-
The root microbiome: Community assembly and its contributions to plant fitness.J Integr Plant Biol. 2022 Feb;64(2):230-243. doi: 10.1111/jipb.13226. J Integr Plant Biol. 2022. PMID: 35029016 Review.
-
Contribution of bacterial-fungal balance to plant and animal health.Curr Opin Microbiol. 2019 Jun;49:66-72. doi: 10.1016/j.mib.2019.10.009. Epub 2019 Nov 12. Curr Opin Microbiol. 2019. PMID: 31731228 Review.
Cited by
-
Impact of conservation tillage on wheat performance and its microbiome.Front Plant Sci. 2023 Aug 21;14:1211758. doi: 10.3389/fpls.2023.1211758. eCollection 2023. Front Plant Sci. 2023. PMID: 37670872 Free PMC article.
-
Arbuscular mycorrhizal fungal spore communities and co-occurrence networks demonstrate host-specific variation throughout the growing season.Mycorrhiza. 2024 Nov;34(5-6):463-475. doi: 10.1007/s00572-024-01168-2. Epub 2024 Sep 18. Mycorrhiza. 2024. PMID: 39292437 Free PMC article.
-
Distribution of rhizosphere fungi of Kobresia humilis on the Qinghai-Tibet Plateau.PeerJ. 2024 Feb 20;12:e16620. doi: 10.7717/peerj.16620. eCollection 2024. PeerJ. 2024. PMID: 38406296 Free PMC article.
-
Life at the borderlands: microbiomes of interfaces critical to One Health.FEMS Microbiol Rev. 2024 Mar 1;48(2):fuae008. doi: 10.1093/femsre/fuae008. FEMS Microbiol Rev. 2024. PMID: 38425054 Free PMC article. Review.
-
Microbiome-enabled genomic selection improves prediction accuracy for nitrogen-related traits in maize.G3 (Bethesda). 2024 Mar 6;14(3):jkad286. doi: 10.1093/g3journal/jkad286. G3 (Bethesda). 2024. PMID: 38113533 Free PMC article.
References
-
- Ahkami A. H., White R., Handakumbura P. P., Jansson C. (2017). Rhizosphere engineering: enhancing sustainable plant ecosystem productivity. Rhizosphere 3, 233–243. doi: 10.1016/J.RHISPH.2017.04.012 - DOI
-
- Aleklett K., Hart M. (2013). The root microbiota-a fingerprint in the soil? Plant Soil 370, 671–686. doi: 10.1007/s11104-013-1647-7 - DOI
-
- Anderson M.J. (2005). PERMANOVA: A FORTRAN Computer Program for Permutational Multivariate Analysis of Variance. Department of Statistics, University of Auckland, Auckland, New Zealand.
Associated data
LinkOut - more resources
Full Text Sources

