NAG-PEGylated multilamellar liposomes for BBB-GLUT transporter targeting
- PMID: 35531302
- PMCID: PMC9075700
- DOI: 10.1080/2331205x.2019.1701343
NAG-PEGylated multilamellar liposomes for BBB-GLUT transporter targeting
Abstract
The primary objective of the research study is to investigate Glucose (GLUT) transporter targeting of the drug (Citalopram-Hbr) for increased permeability across the Blood-Brain Barrier (BBB). The current study reports the development, physicochemical characterization, cytotoxicity analysis and in-vitro BBB permeability assessment of the Citalopram-Hbr liposomal formulations. Rat Primary Brain Microvascular Endothelial Cells (RPBECs) were used for cytotoxicity analysis and drug permeability testing. Five N-Acetyl Glucosamine (NAG) coated PEGylated multilamellar liposomal formulations were prepared and tested. Permeability of the liposomal formulations was evaluated in RPBECs monolayer. The particle size of the formulations ranged from 13 to 4259 nm. Entrapment efficiency was 50-75%. Cytotoxicity analysis indicated viability (>90%) for all five formulations (0.3-1.25 mg/ml). Apparent drug permeability (Papp) of the formulations ranged from 5.01 × 104 to 15 × 104 cm/min. The study demonstrated successful preparation of NAG-coated PEGylated multilamellar liposomal formulations with high drug entrapment efficiency. Cytotoxicity data indicated that the formulations were well tolerated by the cells up to a concentration of 1.25 mg/ml. Transport study data demonstrated that RPBMECs monolayers can be employed as a robust screening tool for future drug transport studies targeting GLUT transporter on the BBB. The drug permeability values provide a promising preliminarily proof that NAG-coated liposomal formulations can be an effective tool for BBB-GLUT transporter targeting.
Keywords: Analysis & Pharmaceutical Quality; Biopharmaceutics; Blood-Brain Barrier (BBB); Drug Design & Development; Drug Discovery; Glucose (GLUT) Transporter; N-acetyl Glucosamine (NAG); Pharmaceutical Science; Pharmacy; liposomes; transporter targeted drug delivery.
Figures

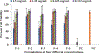



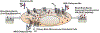
Similar articles
-
QbD approach to investigate product and process variabilities for brain targeting liposomes.J Liposome Res. 2015 Sep;25(3):175-190. doi: 10.3109/08982104.2014.968854. Epub 2014 Oct 13. J Liposome Res. 2015. PMID: 25308415
-
PEGylated Liposomes of Meloxicam: Optimization by Quality by Design, in vitro Characterization and Cytotoxicity Evaluation.Pharm Nanotechnol. 2017;5(2):119-137. doi: 10.2174/2211738505666170428152129. Pharm Nanotechnol. 2017. PMID: 28462699
-
Tamoxifen in topical liposomes: development, characterization and in-vitro evaluation.J Pharm Pharm Sci. 2004 Jul 16;7(2):252-9. J Pharm Pharm Sci. 2004. PMID: 15367383
-
[Development of DDS Actively to Overcome the Blood-brain Barrier in the Region of Ischemic Stroke].Yakugaku Zasshi. 2020;140(8):1007-1012. doi: 10.1248/yakushi.20-00012-7. Yakugaku Zasshi. 2020. PMID: 32741858 Review. Japanese.
-
Revisiting nanoparticle technology for blood-brain barrier transport: Unfolding at the endothelial gate improves the fate of transferrin receptor-targeted liposomes.J Control Release. 2016 Jan 28;222:32-46. doi: 10.1016/j.jconrel.2015.11.032. Epub 2015 Dec 3. J Control Release. 2016. PMID: 26658072 Review.
References
-
- Borlongan CVAE, & Emerich DF (2003). Facilitation of drug entry into the CNS via transient permeation of blood brain barrier: Laboratory and preliminary clinical evidence from bradykinin receptor agonist, Cereport. Brain Research Bulletin, 60(3), 297–306. doi:10.1016/S0361-9230(03)00043-1 - DOI - PubMed
-
- Erdlenbruch B, Alipour M, Fricker G, Miller DS, Kugler W, Eibl H, & Lakomek M (2003). Alkylglycerol opening of the blood-brain barrier to small and large fluorescence markers in normal and C6 glioma-bearing rats and isolated rat brain capillaries. British Journal of Pharmacology, 140(7), 1201–1210. doi:10.1038/sj.bjp.0705554 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
